The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment
- PMID: 30655604
- PMCID: PMC6756114 (VSports app下载)
- DOI: 10.1038/s41388-019-0684-y
The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment
Abstract
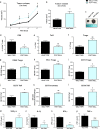
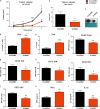
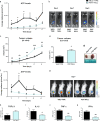
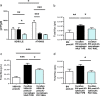
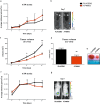
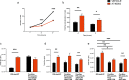
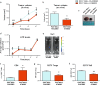
In the tumor microenvironment (TME) ATP and its receptor P2X7 exert a pivotal influence on cancer growth and tumor-host interactions. Here we analyzed the different effect of P2X7 genetic deficiency versus its antagonism on response against P2X7-expressing implanted tumors. We focused on immune cell expression of ATP degrading enzymes CD39 and CD73 and in vivo measured TME's ATP. The immune infiltrate of tumors growing in P2X7 null mice shows a decrease in CD8+ cells and an increased number of Tregs, overexpressing the fitness markers OX40, PD-1, and CD73. A similar Treg phenotype is also present in the spleen of tumor-bearing P2X7 null mice and it is paralleled by a decrease in proinflammatory cytokines and an increase in TGF-β VSports手机版. Differently, systemic administration of the P2X7 blocker A740003 in wild-type mice left unaltered the number of tumor-infiltrating CD8+ and Treg lymphocytes but increased CD4+ effector cells and decreased their expression of CD39 and CD73. P2X7 blockade did not affect spleen immune cell composition or ectonucleotidase expression but increased circulating INF-γ. Augmented CD73 in P2X7 null mice was mirrored by a decrease in TME ATP concentration and nucleotide reduced secretion from immune cells. On the contrary, TME ATP levels remained unaltered upon P2X7 antagonism, owing to release of ATP from cancerous cells and diminished ectonucleotidase expression by CD4+ and dendritic cells. These data point at P2X7 receptor as a key determinant of TME composition due to its combined action on immune cell infiltrate, ectonucleotidases, and ATP release. .
Conflict of interest statement (VSports在线直播)
Professor FDV is a member of the Scientific Advisory Board of Biosceptre Ltd, a Biotech Company involved in the development of P2X7-targeted therapies. The remaining authors declare that they have no conflict of interest V体育安卓版.
"V体育平台登录" Figures

References
-
- Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: a main player in inflammation. Biochem Pharmacol. 2018;151:234–44. doi: 10.1016/j.bcp.2017.12.021. - "VSports手机版" DOI - PubMed
Publication types
- "VSports最新版本" Actions
MeSH terms
- "VSports app下载" Actions
- VSports - Actions
- V体育平台登录 - Actions
- "V体育官网" Actions
- Actions (V体育2025版)
Substances
- "VSports注册入口" Actions
- "V体育官网" Actions
- Actions (V体育安卓版)
- VSports在线直播 - Actions
- Actions (VSports)
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources (V体育平台登录)
Other Literature Sources
Research Materials

