"V体育平台登录" Cell metabolism regulates integrin mechanosensing via an SLC3A2-dependent sphingolipid biosynthesis pathway
- PMID: 30451822
- PMCID: "VSports注册入口" PMC6242995
- DOI: 10.1038/s41467-018-07268-w
Cell metabolism regulates integrin mechanosensing via an SLC3A2-dependent sphingolipid biosynthesis pathway
Abstract
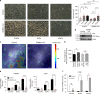
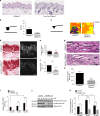
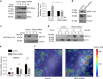
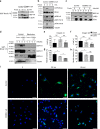
Mechanical and metabolic cues independently contribute to the regulation of cell and tissue homeostasis. However, how they cross-regulate each other during this process remains largely unknown. Here, we show that cellular metabolism can regulate integrin rigidity-sensing via the sphingolipid metabolic pathway controlled by the amino acid transporter and integrin coreceptor CD98hc (SLC3A2). Genetic invalidation of CD98hc in dermal cells and tissue impairs rigidity sensing and mechanical signaling downstream of integrins, including RhoA activation, resulting in aberrant tissue mechanical homeostasis. Unexpectedly, we found that this regulation does not occur directly through regulation of integrins by CD98hc but indirectly, via the regulation of sphingolipid synthesis and the delta-4-desaturase DES2 VSports手机版. Loss of CD98hc decreases sphingolipid availability preventing proper membrane recruitment, shuttling and activation of upstream regulators of RhoA including Src kinases and GEF-H1. Altogether, our results unravel a novel cross-talk regulation between integrin mechanosensing and cellular metabolism which may constitute an important new regulatory framework contributing to mechanical homeostasis. .
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. - "V体育平台登录" DOI - PMC - PubMed
Publication types
- Actions (V体育安卓版)
MeSH terms
- "V体育官网" Actions
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- Actions (VSports app下载)
- VSports注册入口 - Actions
- "VSports手机版" Actions
- Actions (V体育官网)
- VSports最新版本 - Actions
- V体育平台登录 - Actions
Substances
- "V体育ios版" Actions
- Actions (VSports app下载)
- "V体育官网" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
- "V体育官网" Actions
- V体育2025版 - Actions
Grants and funding
LinkOut - more resources
VSports app下载 - Full Text Sources
Molecular Biology Databases
Miscellaneous

