"V体育ios版" Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways
- PMID: 30279690
- PMCID: PMC6153332
- DOI: 10.3389/fimmu.2018.02036
Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways
Abstract
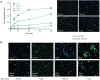
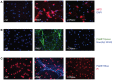
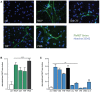
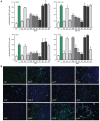
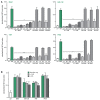
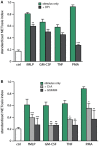
Neutrophils are known to extrude decondensed chromatin, thus forming NETs (neutrophil extracellular traps). These structures immobilize pathogens, thereby preventing their spreading, and are also adorned with antimicrobial molecules. NETs can also influence pathogenesis in chronic inflammation, autoimmunity, and cancer. Despite the importance of NETs, the molecular mechanisms underlying their formation, as well as the upstream signaling pathways involved, are only partially understood. Likewise, current methodological approaches to quantify NETs suffer from significant drawbacks, not the least being the inclusion of a significant non-specific signal VSports手机版. In this study, we used novel, fluorescent polymers that only bind extruded chromatin, allowing a specific and standardized quantification of NETosis. This allowed us to reliably rank the relative potency of various physiologic NET inducers. In neutrophils activated with such stimuli, inhibition of the Syk or PI3K pathways blocked NETosis by acting upon late events in NET formation. Inhibition of the TAK1, p38 MAPK, or MEK pathways also hindered NETosis, but by acting on early events. By contrast, inhibiting PKC, Src family kinases, or JNK failed to prevent NETosis; cycloheximide or actinomycin D were also ineffective. Expectedly, NET formation was deeply compromised following inhibition of the NADPH oxidase in PMA-activated neutrophils, but was found to be ROS-independent in response to physiological agonists. Conversely, we show for the first time in human neutrophils that selective inhibition of PAD4 potently prevents NETosis by all stimuli tested. Our data substantially extends current knowledge of the signaling pathways controlling NETosis, and reveals how they affect early or late stages of the phenomenon. In view of the involvement of NETs in several pathologies, our findings also identify molecular targets that could be exploited for therapeutic intervention. .
Keywords: NADPH oxidase; extracellular traps; neutrophils; protein arginine deiminase; signaling. V体育安卓版.
Figures
References
-
- McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe (2012) 12:324–33. 10.1016/j.chom.2012.06.011 - "V体育安卓版" DOI - PubMed
Publication types
- "V体育官网入口" Actions
MeSH terms
- VSports在线直播 - Actions
- "V体育2025版" Actions
- "V体育安卓版" Actions
- Actions (VSports注册入口)
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- "VSports手机版" Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
Substances
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- Actions (VSports在线直播)
"VSports手机版" Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

