Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps (V体育2025版)
- PMID: 30234114
- PMCID: PMC6131573
- DOI: 10.3389/fcell.2018.00109
"VSports在线直播" Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps
Abstract
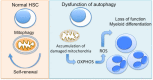
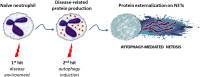
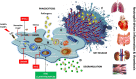
Autophagy is an evolutionarily conserved intracellular degradation system aiming to maintain cell homeostasis in response to cellular stress. At physiological states, basal or constitutive level of autophagy activity is usually low; however, it is markedly up-regulated in response to oxidative stress, nutrient starvation, and various immunological stimuli including pathogens. Many studies over the last years have indicated the implication of autophagy in a plethora of cell populations and functions. In this review, we focus on the role of autophagy in the biology of neutrophils. Early studies provided a link between autophagy and neutrophil cell death, a process essential for resolution of inflammation. Since then, several lines of evidence both in the human system and in murine models propose a critical role for autophagy in neutrophil-driven inflammation and defense against pathogens. Autophagy is essential for major neutrophil functions, including degranulation, reactive oxygen species production, and release of neutrophil extracellular traps VSports手机版. Going back to neutrophil generation in the bone marrow, autophagy plays a critical role in myelopoiesis, driving the differentiation of progenitor cells of the myeloid lineage toward neutrophils. Taken together, in this review we discuss the functional role of autophagy in neutrophils throughout their life, from their production in the bone marrow to inflammatory responses and NETotic cell death. .
Keywords: autophagy; degranulation; granulopoiesis; inflammation; neutrophil; neutrophil extracellular traps; phagocytosis. V体育安卓版.
Figures
References
-
- Akdis C. A., Ballas Z. K. (2017). The editors’ choice. lessons from familial Mediterranean fever: REDD1 is a novel regulator of stress-induced neutrophilic inflammation. J. Allergy Clin. Immunol. 140 1268–1269. 10.1016/j.jaci.2017.09.007 - DOI
-
- Apostolidou E., Skendros P., Kambas K., Mitroulis I., Konstantinidis T., Chrysanthopoulou A., et al. (2016). Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann. Rheum. Dis. 75 269–277. 10.1136/annrheumdis-2014-205958 - V体育官网入口 - DOI - PubMed
-
- Backer J. M. (2016). The intricate regulation and complex functions of the class III phosphoinositide 3-kinase Vps34. Biochem. J. 473 2251–2271. 10.1042/BCJ20160170 - DOI (V体育平台登录) - PubMed
Publication types
LinkOut - more resources
"V体育安卓版" Full Text Sources
V体育2025版 - Other Literature Sources

