Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver
- PMID: 30215866
- PMCID: PMC6460475
- DOI: 10.1002/cphy.c170045
"V体育官网" Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver
Abstract
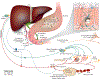
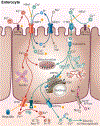
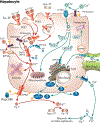
Iron and copper have similar physiochemical properties; thus, physiologically relevant interactions seem likely. Indeed, points of intersection between these two essential trace minerals have been recognized for many decades, but mechanistic details have been lacking. Investigations in recent years have revealed that copper may positively influence iron homeostasis, and also that iron may antagonize copper metabolism. For example, when body iron stores are low, copper is apparently redistributed to tissues important for regulating iron balance, including enterocytes of upper small bowel, the liver, and blood. Copper in enterocytes may positively influence iron transport, and hepatic copper may enhance biosynthesis of a circulating ferroxidase, ceruloplasmin, which potentiates iron release from stores. Moreover, many intestinal genes related to iron absorption are transactivated by a hypoxia-inducible transcription factor, hypoxia-inducible factor-2α (HIF2α), during iron deficiency. Interestingly, copper influences the DNA-binding activity of the HIF factors, thus further exemplifying how copper may modulate intestinal iron homeostasis VSports手机版. Copper may also alter the activity of the iron-regulatory hormone hepcidin. Furthermore, copper depletion has been noted in iron-loading disorders, such as hereditary hemochromatosis. Copper depletion may also be caused by high-dose iron supplementation, raising concerns particularly in pregnancy when iron supplementation is widely recommended. This review will cover the basic physiology of intestinal iron and copper absorption as well as the metabolism of these minerals in the liver. Also considered in detail will be current experimental work in this field, with a focus on molecular aspects of intestinal and hepatic iron-copper interplay and how this relates to various disease states. © 2018 American Physiological Society. Compr Physiol 8:1433-1461, 2018. .
Copyright © 2018 American Physiological Society. All rights reserved. V体育安卓版.
Figures
References
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275: 19906–19912, 2000. - PubMed
-
- Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135: 680–688, 2008. - PubMed
-
- Alexander RT, Rievaj J, Dimke H. Paracellular calcium transport across renal and intestinal epithelia. Biochem Cell Biol 92: 467–480, 2014. - PubMed
-
- Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis 29: 367–375, 2002. - PubMed
-
- Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol 25: 129–135, 2009. - PubMed
VSports最新版本 - Publication types
- VSports手机版 - Actions
MeSH terms
- V体育官网 - Actions
- V体育平台登录 - Actions
Substances
- Actions (VSports app下载)
Grants and funding
"VSports手机版" LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources
Medical

