Phase Transitions Drive the Formation of Vesicular Stomatitis Virus Replication Compartments
- PMID: 30181255
- PMCID: PMC6123442
- DOI: "VSports最新版本" 10.1128/mBio.02290-17
"VSports最新版本" Phase Transitions Drive the Formation of Vesicular Stomatitis Virus Replication Compartments
Abstract
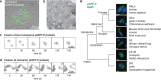
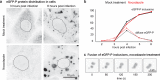
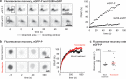
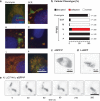
RNA viruses that replicate in the cell cytoplasm typically concentrate their replication machinery within specialized compartments. This concentration favors enzymatic reactions and shields viral RNA from detection by cytosolic pattern recognition receptors. Nonsegmented negative-strand (NNS) RNA viruses, which include some of the most significant human, animal, and plant pathogens extant, form inclusions that are sites of RNA synthesis and are not circumscribed by a membrane. These inclusions share similarities with cellular protein/RNA structures such as P granules and nucleoli, which are phase-separated liquid compartments. Here we show that replication compartments of vesicular stomatitis virus (VSV) have the properties of liquid-like compartments that form by phase separation. Expression of the individual viral components of the replication machinery in cells demonstrates that the 3 viral proteins required for replication are sufficient to drive cytoplasmic phase separation. Therefore, liquid-liquid phase separation, previously linked to organization of P granules, nucleolus homeostasis, and cell signaling, plays a key role in host-pathogen interactions. This work suggests novel therapeutic approaches to the problem of combating NNS RNA viral infections VSports手机版. IMPORTANCE RNA viruses compartmentalize their replication machinery to evade detection by host pattern recognition receptors and concentrate the machinery of RNA synthesis. For positive-strand RNA viruses, RNA replication occurs in a virus-induced membrane-associated replication organelle. For NNS RNA viruses, the replication compartment is a cytoplasmic inclusion that is not circumscribed by a cellular membrane. Such structures were first observed in the cell bodies of neurons from humans infected with rabies virus and were termed Negri bodies. How the replication machinery that forms this inclusion remains associated in the absence of a membrane has been an enduring mystery. In this article, we present evidence that the VSV replication compartments form through phase separation. Phase separation is increasingly recognized as responsible for cellular structures as diverse as processing bodies (P-bodies) and nucleoli and was recently demonstrated for rabies virus. This article further links the fields of host-pathogen interaction with that of phase separation. .
Keywords: negative-strand RNA virus; phase separation; rhabdovirus; viral replication; viroplasm; virus-host interactions. V体育安卓版.
Copyright © 2018 Heinrich et al.
Figures
References
V体育平台登录 - Publication types
MeSH terms
- "V体育安卓版" Actions
- "VSports手机版" Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- "VSports在线直播" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
Substances
- "V体育官网入口" Actions
Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources

