"VSports注册入口" The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity
- PMID: 30076299
- PMCID: PMC6076291
- DOI: 10.1038/s41467-018-05395-y
The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity
Abstract
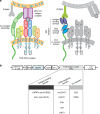
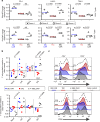
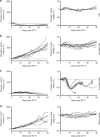
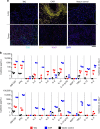
Engineering T cells with chimeric antigen receptors (CARs) is an effective method for directing T cells to attack tumors, but may cause adverse side effects such as the potentially lethal cytokine release syndrome. Here the authors show that the T cell antigen coupler (TAC), a chimeric receptor that co-opts the endogenous TCR, induces more efficient anti-tumor responses and reduced toxicity when compared with past-generation CARs. TAC-engineered T cells induce robust and antigen-specific cytokine production and cytotoxicity in vitro, and strong anti-tumor activity in a variety of xenograft models including solid and liquid tumors. In a solid tumor model, TAC-T cells outperform CD28-based CAR-T cells with increased anti-tumor efficacy, reduced toxicity, and faster tumor infiltration VSports手机版. Intratumoral TAC-T cells are enriched for Ki-67+ CD8+ T cells, demonstrating local expansion. These results indicate that TAC-T cells may have a superior therapeutic index relative to CAR-T cells. .
Conflict of interest statement
Christopher W V体育安卓版. Helsen, Joanne A. Hammill, Kenneth A. Mwawasi, Arya Afsahi, Galina F. Denisova, and Jonathan L. Bramson hold shares in Triumvira Immunologics. Christopher W. Helsen and Jonathan L. Bramson are founding scientists of Triumvira Immunologics. All other authors declare no competing interests.
Figures


References (V体育安卓版)
-
- Ramos CA, Savoldo B, Dotti G. CD19-CAR trials. Cancer J. 2015;20:112–118. doi: 10.1097/PPO.0000000000000031. - "VSports app下载" DOI - PMC - PubMed
-
- Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–2797. - VSports最新版本 - PubMed
Publication types
MeSH terms
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- Actions (VSports手机版)
- Actions (V体育ios版)
- Actions (V体育平台登录)
- "VSports在线直播" Actions
- Actions (V体育官网)
- "VSports app下载" Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
V体育官网 - Substances
- "V体育官网" Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports手机版 - Research Materials
Miscellaneous

