V体育2025版 - Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota
- PMID: 30049384
- PMCID: "V体育安卓版" PMC6085585
- DOI: 10.1016/j.ebiom.2018.06.029
Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota
Abstract
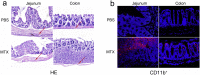
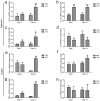
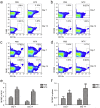
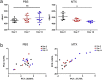
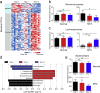
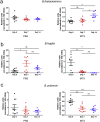
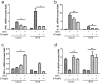
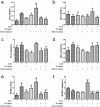
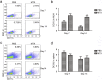
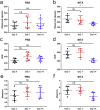
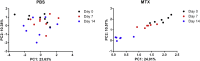
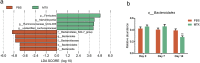
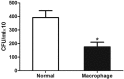
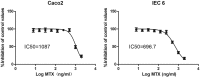
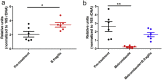
As a widely used anticancer and immunosuppressive agent, methotrexate (MTX) can induce multiple adverse drug reactions (ADRs), such as gastrointestinal toxicity, the mechanisms are poorly understood. Gut microbiota has been widely reported to be associated with the onset of multiple diseases as well as treatment outcomes of different drugs. In this study, mucosal injury was observed in MTX-treated mice, leading to significant changes in macrophages (i. e. , M1/M2 ratio, P < 0. 05) but not in dendritic cells. Moreover, the population, diversity and principal components of the gut microbiota in mice were dramatically altered after MTX treatment in a time-dependent manner, and Bacteroidales exhibited the most distinct variation among all the taxa (P < 0. 05). Bacteroides fragilis was significantly decreased with MTX treatment (P < 0. 01) and tended to decrease proportionately with increasing macrophage density. Gavage of mice with B. fragilis ameliorated MTX-induced inflammatory reactions and modulate macrophage polarization. In conclusion, our results delineate a strong impact of the gut microbiota on MTX-induced intestinal mucositis and provide a potential method for the prevention of such ADRs. VSports手机版.
Keywords: Gastrointestinal toxicity; Gut microbiota; Methotrexate; Mononuclear phagocyte. V体育安卓版.
Copyright © 2018 The Authors. Published by Elsevier B. V V体育ios版. All rights reserved. .
Figures
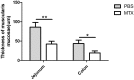
References (VSports最新版本)
-
- Leitao R.F., Brito G.A., Oria R.B., Braga-Neto M.B., Bellaguarda E.A., Silva J.V. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011;11:90. [Epub 2011/08/19] - VSports - PMC - PubMed
-
- Neradil J., Pavlasova G., Veselska R. New mechanisms for an old drug; DHFR- and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol. 2012;25(Suppl. 2) [2S87-92. Epub 2012/01/01] - "VSports注册入口" PubMed
-
- Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146(5):489–503. [Epub 2009/06/23] - V体育2025版 - PubMed
-
- Paci A., Veal G., Bardin C., Leveque D., Widmer N., Beijnen J. Review of therapeutic drug monitoring of anticancer drugs part 1—cytotoxics. Eur J Cancer. 2014;50(12):2010–2019. [Epub 2014/06/04] - PubMed
MeSH terms (V体育官网)
- V体育ios版 - Actions
- "VSports app下载" Actions
- Actions (VSports)
- "VSports注册入口" Actions
- Actions (V体育官网)
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- "V体育官网" Actions
- VSports最新版本 - Actions
- Actions (V体育平台登录)
- "VSports app下载" Actions
- "V体育官网入口" Actions
Substances
- Actions (VSports手机版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (VSports手机版)
Research Materials

