From proteomics to discovery of first-in-class ST2 inhibitors active in vivo
- PMID: 30046004
- PMCID: "V体育2025版" PMC6124397
- DOI: 10.1172/jci.insight.99208
From proteomics to discovery of first-in-class ST2 inhibitors active in vivo
Abstract (V体育2025版)
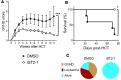
Soluble cytokine receptors function as decoy receptors to attenuate cytokine-mediated signaling and modulate downstream cellular responses. Dysregulated overproduction of soluble receptors can be pathological, such as soluble ST2 (sST2), a prognostic biomarker in cardiovascular diseases, ulcerative colitis, and graft-versus-host disease (GVHD) VSports手机版. Although intervention using an ST2 antibody improves survival in murine GVHD models, sST2 is a challenging target for drug development because it binds to IL-33 via an extensive interaction interface. Here, we report the discovery of small-molecule ST2 inhibitors through a combination of high-throughput screening and computational analysis. After in vitro and in vivo toxicity assessment, 3 compounds were selected for evaluation in 2 experimental GVHD models. We show that the most effective compound, iST2-1, reduces plasma sST2 levels, alleviates disease symptoms, improves survival, and maintains graft-versus-leukemia activity. Our data suggest that iST2-1 warrants further optimization to develop treatment for inflammatory diseases mediated by sST2. .
Keywords: Drug screens; Stem cell transplantation; Th2 response; Therapeutics; Transplantation V体育安卓版. .
VSports注册入口 - Conflict of interest statement
Figures
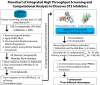
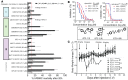
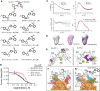

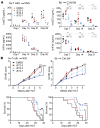
References
Publication types
- Actions (V体育官网入口)
- "VSports手机版" Actions
V体育安卓版 - MeSH terms
- V体育ios版 - Actions
- Actions (V体育2025版)
- V体育安卓版 - Actions
- Actions (VSports app下载)
- Actions (V体育官网入口)
- "VSports最新版本" Actions
- V体育平台登录 - Actions
Substances
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- Actions (V体育平台登录)
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育ios版" Other Literature Sources

