V体育2025版 - D-2-Hydroxyglutarate Is an Intercellular Mediator in IDH-Mutant Gliomas Inhibiting Complement and T Cells
- PMID: 30006485
- PMCID: PMC6214730 (V体育安卓版)
- DOI: 10.1158/1078-0432.CCR-17-3855 (VSports最新版本)
"VSports最新版本" D-2-Hydroxyglutarate Is an Intercellular Mediator in IDH-Mutant Gliomas Inhibiting Complement and T Cells
"V体育ios版" Abstract
Purpose: Somatic mutations in the isocitrate dehydrogenase (IDH)-1 and -2 genes are remarkably penetrant in diffuse gliomas. These highly effective gain-of-function mutations enable mutant IDH to efficiently metabolize isocitrate to D-2-hydroxyglutarate (D 2-HG) that accumulates to high concentrations within the tumor microenvironment. D 2-HG is an intracellular effector that promotes tumor growth through widespread epigenetic changes in IDH-mutant tumor cells, but its potential role as an intercellular immune regulator remains understudied. Experimental Design: Complement activation and CD4+, CD8+, or FOXP3+ T-cell infiltration into primary tumor tissue were determined by immunohistochemistry using sections from 72 gliomas of World Health Organization (WHO) grade III and IV with or without IDH mutations. Ex vivo experiments with D 2-HG identified immune inhibitory mechanisms VSports手机版. Results: IDH mutation associated with significantly reduced complement activation and decreased numbers of tumor-infiltrating CD4+ and CD8+ T cells with comparable FOXP3+/CD4+ ratios. D 2-HG potently inhibited activation of complement by the classical and alternative pathways, attenuated complement-mediated glioma cell damage, decreased cellular C3b(iC3b) opsonization, and impaired complement-mediated phagocytosis. Although D 2-HG did not affect dendritic cell differentiation or function, it significantly inhibited activated T-cell migration, proliferation, and cytokine secretion. Conclusions: D 2-HG suppresses the host immune system, potentially promoting immune escape of IDH-mutant tumors. Clin Cancer Res; 24(21); 5381-91. ©2018 AACR. .
©2018 American Association for Cancer Research. V体育安卓版.
VSports app下载 - Conflict of interest statement
The authors declare no potential conflicts of interest
Figures

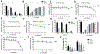



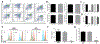
References
-
- Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–18. - VSports注册入口 - PubMed
-
- Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–74. - PubMed
"VSports注册入口" Publication types
- Actions (VSports最新版本)
- "VSports在线直播" Actions
MeSH terms
- "VSports app下载" Actions
- VSports app下载 - Actions
- VSports注册入口 - Actions
- VSports - Actions
- "VSports最新版本" Actions
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- "V体育2025版" Actions
- "VSports" Actions
- Actions (VSports在线直播)
- "V体育2025版" Actions
Substances
- "V体育安卓版" Actions
- Actions (V体育平台登录)
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (V体育官网)
Other Literature Sources (V体育安卓版)
Research Materials
Miscellaneous

