"VSports注册入口" Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma
- PMID: 29939160
- PMCID: PMC6063467
- DOI: "V体育官网" 10.1172/JCI99032
Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma
VSports - Abstract
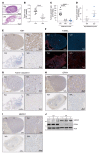
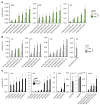
High-risk neuroblastoma is a devastating malignancy with very limited therapeutic options VSports手机版. Here, we identify withaferin A (WA) as a natural ferroptosis-inducing agent in neuroblastoma, which acts through a novel double-edged mechanism. WA dose-dependently either activates the nuclear factor-like 2 pathway through targeting of Kelch-like ECH-associated protein 1 (noncanonical ferroptosis induction) or inactivates glutathione peroxidase 4 (canonical ferroptosis induction). Noncanonical ferroptosis induction is characterized by an increase in intracellular labile Fe(II) upon excessive activation of heme oxygenase-1, which is sufficient to induce ferroptosis. This double-edged mechanism might explain the superior efficacy of WA as compared with etoposide or cisplatin in killing a heterogeneous panel of high-risk neuroblastoma cells, and in suppressing the growth and relapse rate of neuroblastoma xenografts. Nano-targeting of WA allows systemic application and suppressed tumor growth due to an enhanced accumulation at the tumor site. Collectively, our data propose a novel therapeutic strategy to efficiently kill cancer cells by ferroptosis. .
Keywords: Drug therapy; Nanotechnology; Neurological disorders; Neuroscience; Oncology V体育安卓版. .
Conflict of interest statement (V体育官网)
Figures



References
-
- Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–2211. doi: 10.1056/NEJMra0804577. - DOI (VSports) - PMC - PubMed
-
- Aaes TL, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274–287. doi: 10.1016/j.celrep.2016.03.037. - DOI (VSports手机版) - PubMed
Publication types
- V体育安卓版 - Actions
- Actions (VSports最新版本)
MeSH terms
- "VSports手机版" Actions
- Actions (V体育官网入口)
- V体育2025版 - Actions
- VSports注册入口 - Actions
- VSports最新版本 - Actions
- VSports在线直播 - Actions
VSports app下载 - Substances
- Actions (V体育官网)
- "V体育安卓版" Actions
- VSports手机版 - Actions
- VSports最新版本 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (V体育2025版)
"VSports" Other Literature Sources
Medical (VSports)
Molecular Biology Databases
Research Materials

