Fibroblast growth factor 23 and Klotho contribute to airway inflammation
- PMID: 29748308
- PMCID: PMC6044452
- DOI: 10.1183/13993003.00236-2018
Fibroblast growth factor 23 and Klotho contribute to airway inflammation
Abstract
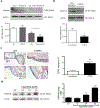
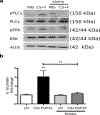
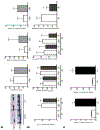
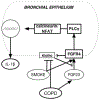
Circulating levels of fibroblast growth factor (FGF)23 are associated with systemic inflammation and increased mortality in chronic kidney disease. α-Klotho, a co-receptor for FGF23, is downregulated in chronic obstructive pulmonary disease (COPD). However, whether FGF23 and Klotho-mediated FGF receptor (FGFR) activation delineates a pathophysiological mechanism in COPD remains unclear. We hypothesised that FGF23 can potentiate airway inflammation via Klotho-independent FGFR4 activation. FGF23 and its effect were studied using plasma and transbronchial biopsies from COPD and control patients, and primary human bronchial epithelial cells isolated from COPD patients as well as a murine COPD model. Plasma FGF23 levels were significantly elevated in COPD patients. Exposure of airway epithelial cells to cigarette smoke and FGF23 led to a significant increase in interleukin-1β release via Klotho-independent FGFR4-mediated activation of phospholipase Cγ/nuclear factor of activated T-cells signalling. In addition, Klotho knockout mice developed COPD and showed airway inflammation and elevated FGFR4 expression in their lungs, whereas overexpression of Klotho led to an attenuation of airway inflammation. Cigarette smoke induces airway inflammation by downregulation of Klotho and activation of FGFR4 in the airway epithelium in COPD. Inhibition of FGF23 or FGFR4 might serve as a novel anti-inflammatory strategy in COPD. VSports手机版.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: None declared.
Figures




References
-
- Samet JM (2013) Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin 23, 103–112 - PubMed
-
- de Boer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken JH, and Hiemstra PS (2000) Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol 190, 619–626 - PubMed
-
- Polosukhin VV (2001) Ultrastructural of the bronchial epithelium in chronic inflammation. Ultrastruct Pathol 25, 119–128 - PubMed (V体育平台登录)
-
- Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, and Folkerts G (2011) Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochimica et biophysica acta 1812, 1104–1110 - PubMed
-
- Schiavi SC, and Kumar R (2004) The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int 65, 1–14 - PubMed
"V体育ios版" Publication types
- Actions (V体育官网入口)
"VSports最新版本" MeSH terms
- "VSports" Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
- "VSports注册入口" Actions
- VSports手机版 - Actions
- V体育平台登录 - Actions
- "V体育ios版" Actions
- V体育官网入口 - Actions
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- "V体育2025版" Actions
Substances
- V体育ios版 - Actions
- "VSports手机版" Actions
- V体育官网 - Actions
Grants and funding (V体育ios版)
LinkOut - more resources
Full Text Sources
"VSports手机版" Other Literature Sources
VSports在线直播 - Medical
Miscellaneous
