"V体育平台登录" Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation
- PMID: 29703902
- PMCID: PMC5923228
- DOI: 10.1038/s41467-018-03966-7
Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation
"VSports在线直播" Abstract
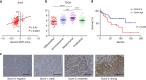
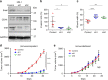
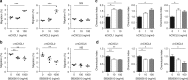
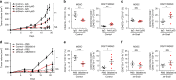
Snail is a major transcriptional factor that induces epithelial-mesenchymal transition (EMT) VSports手机版. In this study, we explore the effect of Snail on tumor immunity. Snail knockdown in mouse ovarian cancer cells suppresses tumor growth in immunocompetent mice, associated with an increase of CD8+ tumor-infiltrating lymphocytes and a decrease of myeloid-derived suppressor cells (MDSCs). Snail knockdown reduces the expression of CXCR2 ligands (CXCL1 and CXCL2), chemokines that attract MDSCs to the tumor via CXCR2. Snail upregulates CXCR ligands through NF-kB pathway, and most likely, through direct binding to the promoters. A CXCR2 antagonist suppresses MDSC infiltration and delays tumor growth in Snail-expressing mouse tumors. Ovarian cancer patients show elevated serum CXCL1/2, which correlates with Snail expression, MDSC infiltration, and short overall survival. Thus, Snail induces cancer progression via upregulation of CXCR2 ligands and recruitment of MDSCs. Blocking CXCR2 represents an immunological therapeutic approach to inhibit progression of Snail-high tumors undergoing EMT. .
Conflict of interest statement
E. N. was involved in this study as effort outside of the endowed associate professor of DSK project, Medical Innovation Center, Kyoto University sponsored by Sumitomo Dainippon Pharma Co Ltd V体育安卓版. The remaining authors declare no competing interests.
Figures



"V体育安卓版" References
-
- Davidson B, Trope CG, Reich R. Epithelial-mesenchymal transition in ovarian carcinoma. Front Oncol. 2012;2:33. doi: 10.3389/fonc.2012.00033. - "VSports手机版" DOI - PMC - PubMed
-
- Tan TZ, et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 2014;6:1279–1293. doi: 10.15252/emmm.201404208. - "VSports" DOI - PMC - PubMed
Publication types
- "VSports app下载" Actions
MeSH terms
- V体育官网入口 - Actions
- "VSports手机版" Actions
- "VSports" Actions
- Actions (VSports注册入口)
- Actions (VSports注册入口)
- V体育平台登录 - Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
- Actions (VSports在线直播)
- V体育平台登录 - Actions
- Actions (VSports最新版本)
Substances
- Actions (VSports在线直播)
- VSports - Actions
- Actions (V体育官网入口)
- VSports - Actions
- "V体育平台登录" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

