Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis (VSports)
- PMID: 29632885
- PMCID: V体育官网 - PMC5879472
- DOI: "VSports在线直播" 10.1021/acscentsci.7b00589
Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis
Abstract
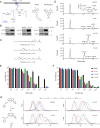
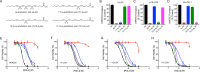
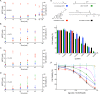
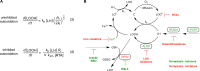
Lipoxygenases (LOXs) have been implicated as central players in ferroptosis, a recently characterized cell death modality associated with the accumulation of lipid hydroperoxides: the products of LOX catalysis. To provide insight on their role, human embryonic kidney cells were transfected to overexpress each of the human isoforms associated with disease, 5-LOX, p12-LOX, and 15-LOX-1, which yielded stable cell lines that were demonstrably sensitized to ferroptosis. Interestingly, the cells could be rescued by less than half of a diverse collection of known LOX inhibitors. Furthermore, the cytoprotective compounds were similarly potent in each of the cell lines even though some were clearly isoform-selective LOX inhibitors. The cytoprotective compounds were subsequently demonstrated to be effective radical-trapping antioxidants, which protect lipids from autoxidation, the autocatalytic radical chain reaction that produces lipid hydroperoxides. From these data (and others reported herein), a picture emerges wherein LOX activity may contribute to the cellular pool of lipid hydroperoxides that initiate ferroptosis, but lipid autoxidation drives the cell death process. VSports手机版.
Conflict of interest statement
The authors declare the following competing financial interest(s): M. S V体育安卓版. S. owns shares in Retrotope, Inc.
"V体育ios版" Figures


References
-
- Angeli J. P. F.; Shah R.; Pratt D. A.; Conrad M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. 10.1016/j.tips.2017.02.005. - VSports - DOI - PubMed
LinkOut - more resources
V体育安卓版 - Full Text Sources
Other Literature Sources

