Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity
- PMID: 29592876
- PMCID: PMC5894265
- DOI: 10.1182/bloodadvances.2018017731
Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity
Abstract
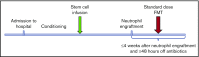
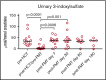
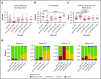
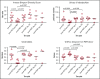
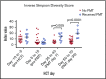
We hypothesized that third-party fecal microbiota transplantation (FMT) may restore intestinal microbiome diversity after allogeneic hematopoietic cell transplantation (allo-HCT). In this open-label single-group pilot study, 18 subjects were enrolled before allo-HCT and planned to receive third-party FMT capsules. FMT capsules were administered no later than 4 weeks after neutrophil engraftment, and antibiotics were not allowed within 48 hours before FMT. Five patients did not receive FMT because of the development of early acute gastrointestinal (GI) graft-versus-host disease (GVHD) before FMT (n = 3), persistent HCT-associated GI toxicity (n = 1), or patient decision (n = 1). Thirteen patients received FMT at a median of 27 days (range, 19-45 days) after HCT. Participants were able to swallow and tolerate all FMT capsules, meeting the primary study endpoint of feasibility. FMT was tolerated well, with 1 treatment-related significant adverse event (abdominal pain). Two patients subsequently developed acute GI GVHD, with 1 patient also having concurrent bacteremia. No additional cases of bacteremia occurred. Median follow-up for survivors is 15 months (range, 13-20 months). The Kaplan-Meier estimates for 12-month overall survival and progression-free survival after FMT were 85% (95% confidence interval, 51%-96%) and 85% (95% confidence interval, 51%-96%), respectively. There was 1 nonrelapse death resulting from acute GI GVHD (12-month nonrelapse mortality, 8%; 95% confidence interval, 0%-30%). Analysis of stool composition and urine 3-indoxyl sulfate concentration indicated improvement in intestinal microbiome diversity after FMT that was associated with expansion of stool-donor taxa. These results indicate that empiric third-party FMT after allo-HCT appears to be feasible, safe, and associated with expansion of recipient microbiome diversity. This trial was registered at www. clinicaltrials. gov as #NCT02733744 VSports手机版. .
© 2018 by The American Society of Hematology. V体育安卓版.
Conflict of interest statement
Conflict-of-interest disclosure: E. Hohmann receives research support from Seres Therapeutics, Inc. J. U. P. holds patents with or receives royalties from Seres Therapeutics, Inc. R. R. J. is on the board of directors or an advisory committee for Seres Therapeutics, Inc. , has consulted for Ziopharm Oncology, and holds patents with or receives royalties from Seres Therapeutics, Inc. M. R. M. v. d. B. is on the board of directors or an advisory committee for Seres Therapeutics, Inc. and holds patents with or receives royalties from Seres Therapeutics, Inc. E. G. P V体育ios版. is on the board of directors or an advisory committee for Seres Therapeutics, Inc. and holds patents with or receives royalties from Seres Therapeutics, Inc. The remaining authors declare no competing financial interests.
Figures


References (VSports最新版本)
-
- Mathewson ND, Reddy P. The microbiome and graft versus host disease. Curr Stem Cell Rep. 2015;1(1):39-47
-
- Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174-1182 - "VSports最新版本" PMC - PubMed
Publication types
- Actions (V体育官网入口)
- V体育官网 - Actions
MeSH terms
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- Actions (VSports手机版)
- Actions (V体育ios版)
- Actions (V体育安卓版)
- Actions (V体育平台登录)
- "VSports注册入口" Actions
- Actions (VSports在线直播)
Associated data
- V体育官网入口 - Actions
"V体育官网" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

