V体育ios版 - Effect of IL-18 on the Expansion and Phenotype of Human Natural Killer Cells: Application to Cancer Immunotherapy
- PMID: 29559850
- PMCID: PMC5859478
- DOI: 10.7150/ijbs.22809
Effect of IL-18 on the Expansion and Phenotype of Human Natural Killer Cells: Application to Cancer Immunotherapy
Abstract
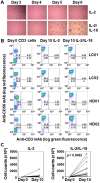
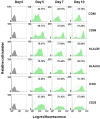
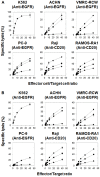
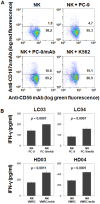
When pathogenic stresses are recognized by innate immune cells, inflammasomes are assembled and caspase-1 is activated, resulting in the conversion of pro-IL-18 into mature IL-18. Because natural killer (NK) cells express IL-18 receptors, IL-18 may play roles in immune functions of NK cells. In the present study, we examined the effect of IL-18 on NK cells derived from lung cancer patients and healthy adult volunteers. When peripheral blood NK cells were stimulated with IL-2, the cells formed clusters beginning on day 5-6 and proliferated thereafter, in which the number of NK cells increased by 10-fold in 10 days. When IL-18 was added, cell clusters were observed as early as on day 4 and NK cells proliferated vigorously. On day 10, the expansion rate was 56-fold on average, showing that IL-18 promoted the expansion of NK cells VSports手机版. It was also notable that IL-18 enhanced the expression of CD80, CD86, HLA-DR and HLA-DQ on NK cells, suggesting that IL-18 conferred NK cells an APC-like phenotype. When cellular cytotoxicity was determined, APC-like NK cells efficiently killed tumor cells and anti-tumor activity was augmented by the addition of tumor antigen-specific mAbs. In addition, IFN-γ was produced by APC-like NK cells in response to tumor cells, and the cytokine production was further enhanced by mAbs. Taken together, IL-18 not only promoted the expansion of NK cells, but also changed the phenotype of NK cells. IL-2/IL-18-induced NK cells might, therefore, serve as a bridge between innate immunity and adaptive immunity and be useful for cancer immunotherapy. .
Keywords: IL-18; NK cells; PD-1; antigen-presenting cells; immune checkpoint. V体育安卓版.
Conflict of interest statement
Competing Interests: YT is a co-inventor of Japanese Patent 2014-73475 on the development of a non-radioactive cellular cytotoxicity assay using a precursor of a novel Eu chelate-forming compound. The other authors have no conflicts of interest V体育ios版.
Figures
References
-
- Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N. et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. - PubMed (V体育ios版)
Publication types
- "V体育官网入口" Actions
MeSH terms
- V体育安卓版 - Actions
- "V体育ios版" Actions
- V体育官网 - Actions
- "VSports最新版本" Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "V体育安卓版" Actions
Substances (VSports在线直播)
- VSports注册入口 - Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

