SWATH mass spectrometry as a tool for quantitative profiling of the matrisome
- PMID: 29501709
- PMCID: V体育平台登录 - PMC6215756
- DOI: 10.1016/j.jprot.2018.02.026 (VSports最新版本)
SWATH mass spectrometry as a tool for quantitative profiling of the matrisome (VSports)
"V体育官网入口" Abstract
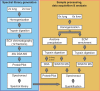
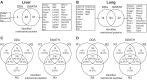
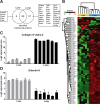
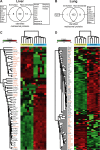
Proteomic analysis of extracellular matrix (ECM) and ECM-associated proteins, collectively known as the matrisome, is a challenging task due to the inherent complexity and insolubility of these proteins VSports手机版. Here we present sequential window acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH MS) as a tool for the quantitative analysis of matrisomal proteins in both non-enriched and ECM enriched tissue without the need for prior fractionation. Utilising a spectral library containing 201 matrisomal proteins, we compared the performance and reproducibility of SWATH MS over conventional data-dependent analysis mass spectrometry (DDA MS) in unfractionated murine lung and liver. SWATH MS conferred a 15-20% increase in reproducible peptide identification across replicate experiments in both tissue types and identified 54% more matrisomal proteins in the liver versus DDA MS. We further use SWATH MS to evaluate the quantitative changes in matrisome content that accompanies ECM enrichment. Our data shows that ECM enrichment led to a systematic increase in core matrisomal proteins but resulted in significant losses in matrisome-associated proteins including the cathepsins and proteins of the S100 family. Our proof-of-principle study demonstrates the utility of SWATH MS as a versatile tool for in-depth characterisation of the matrisome in unfractionated and non-enriched tissues. SIGNIFICANCE: The matrisome is a complex network of extracellular matrix (ECM) and ECM-associated proteins that provides scaffolding function to tissues and plays important roles in the regulation of fundamental cellular processes. However, due to its inherent complexity and insolubility, proteomic studies of the matrisome typically require the application of enrichment workflows prior to MS analysis. Such enrichment strategies often lead to losses in soluble matrisome-associated components. In this study, we present sequential window acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH MS) as a tool for the quantitative analysis of matrisomal proteins. We show that SWATH MS provides a more reproducible coverage of the matrisome compared to data-dependent analysis (DDA) MS. We also demonstrate that SWATH MS is capable of accurate quantification of matrisomal proteins without prior ECM enrichment and fractionation, which may simplify sample handling workflows and avoid losses in matrisome-associated proteins commonly linked to ECM enrichment. .
Keywords: DIA MS; Extracellular matrix; Mass spectrometry; Matrisome; Proteomics; SWATH MS. V体育安卓版.
Copyright © 2018 The Authors. Published by Elsevier B. V. All rights reserved V体育ios版. .
Figures

References (VSports注册入口)
-
- Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. - V体育ios版 - PMC - PubMed
-
- Chu M.L., Williams C.J., Pepe G., Hirsch J.L., Prockop D.J., Ramirez F. Internal deletion in a collagen GENE in a perinatal lethal form of osteogenesis imperfecta. Nature. 1983;304:78–80. - PubMed
-
- Hudson B.G., Tryggvason K., Sundaramoorthy M., Neilson E.G. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N. Engl. J. Med. 2003;348:2543–2556. - PubMed
Publication types
- "V体育官网" Actions
"VSports注册入口" MeSH terms
- Actions (V体育官网入口)
- Actions (V体育安卓版)
- Actions (VSports app下载)
- "VSports手机版" Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
Substances
"VSports注册入口" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

