VSports手机版 - ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages
- PMID: 29491424
- PMCID: "V体育安卓版" PMC5830643
- DOI: 10.1038/s41598-018-21998-3
"V体育平台登录" ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages
Abstract
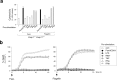
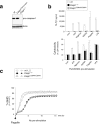
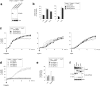
The NLRC4 inflammasome recognizes bacterial flagellin and components of the type III secretion apparatus. NLRC4 stimulation leads to caspase-1 activation followed by a rapid lytic cell death known as pyroptosis. NLRC4 is linked to pathogen-free auto-inflammatory diseases, suggesting a role for NLRC4 in sterile inflammation. Here, we show that NLRC4 activates an alternative cell death program morphologically similar to apoptosis in caspase-1-deficient BMDMs. By performing an unbiased genome-wide CRISPR/Cas9 screen with subsequent validation studies in gene-targeted mice, we highlight a critical role for caspase-8 and ASC adaptor in an alternative apoptotic pathway downstream of NLRC4. Furthermore, caspase-1 catalytically dead knock-in (Casp1 C284A KI) BMDMs genetically segregate pyroptosis and apoptosis, and confirm that caspase-1 does not functionally compete with ASC for NLRC4 interactions VSports手机版. We show that NLRC4/caspase-8-mediated apoptotic cells eventually undergo plasma cell membrane damage in vitro, suggesting that this pathway can lead to secondary necrosis. Unexpectedly, we found that DFNA5/GSDME, a member of the pore-forming gasdermin family, is dispensable for the secondary necrosis that follows NLRC4-mediated apoptosis in macrophages. Together, our data confirm the existence of an alternative caspase-8 activation pathway diverging from the NLRC4 inflammasome in primary macrophages. .
Conflict of interest statement
The authors declare no competing interests.
Figures (VSports最新版本)



References
-
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. - "V体育安卓版" DOI - PubMed
VSports注册入口 - MeSH terms
- Actions (V体育安卓版)
- Actions (VSports app下载)
- V体育ios版 - Actions
- "V体育官网入口" Actions
- Actions (V体育官网)
- Actions (VSports在线直播)
Substances
- Actions (V体育平台登录)
- "V体育官网" Actions
- VSports最新版本 - Actions
- VSports注册入口 - Actions
LinkOut - more resources
"V体育平台登录" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
"VSports app下载" Miscellaneous

