COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series)
- PMID: 29468936
- PMCID: PMC5826015
- DOI: "V体育ios版" 10.1177/2045894018758528
"V体育官网" COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series)
Abstract
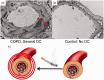
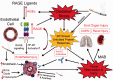
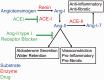
Chronic obstructive pulmonary disease (COPD) is characterized by chronic expiratory airflow obstruction that is not fully reversible. COPD patients develop varying degrees of emphysema, small and large airway disease, and various co-morbidities VSports手机版. It has not been clear whether these co-morbidities share common underlying pathogenic processes with the pulmonary lesions. Early research into the pathogenesis of COPD focused on the contributions of injury to the extracellular matrix and pulmonary epithelial cells. More recently, cigarette smoke-induced endothelial dysfunction/injury have been linked to the pulmonary lesions in COPD (especially emphysema) and systemic co-morbidities including atherosclerosis, pulmonary hypertension, and chronic renal injury. Herein, we review the evidence linking endothelial injury to COPD, and the pathways underlying endothelial injury and the "vascular COPD phenotype" including: (1) direct toxic effects of cigarette smoke on endothelial cells; (2) generation of auto-antibodies directed against endothelial cells; (3) vascular inflammation; (4) increased oxidative stress levels in vessels inducing increases in lipid peroxidation and increased activation of the receptor for advanced glycation end-products (RAGE); (5) reduced activation of the anti-oxidant pathways in endothelial cells; (6) increased endothelial cell release of mediators with vasoconstrictor, pro-inflammatory, and remodeling activities (endothelin-1) and reduced endothelial cell expression of mediators that promote vasodilation and homeostasis of endothelial cells (nitric oxide synthase and prostacyclin); and (7) increased endoplasmic reticular stress and the unfolded protein response in endothelial cells. We also review the literature on studies of drugs that inhibit RAGE signaling in other diseases (angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), or vasodilators developed for idiopathic pulmonary arterial hypertension that have been tested on cell culture systems, animal models of COPD, and/or smokers and COPD patients. .
Keywords: RAGE; apoptosis; oxidative stress; pulmonary endothelium; renal injury. V体育安卓版.
Figures

References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. - PubMed
-
- Torres-Duque C, Maldonado D, Perez-Padilla R, et al. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc 2008; 5: 577–590. - PubMed
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370: 765–773. - V体育官网入口 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

