Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22
- PMID: 29459771
- PMCID: PMC6143595
- DOI: 10.1038/s41418-018-0070-2
Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22
Erratum in
-
Correction: Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22.Cell Death Differ. 2021 Jun;28(6):2025-2027. doi: 10.1038/s41418-020-00630-w. Cell Death Differ. 2021. PMID: 33087876 Free PMC article. No abstract available.
Abstract
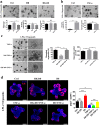
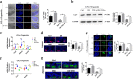
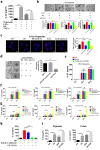
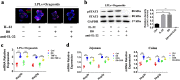
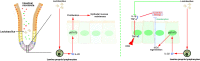
The regeneration of intestinal epithelial are maintained by continuous differentiation and proliferation of intestinal stem cells (ISCs) under physiological and pathological conditions. However, little is known about the regulatory effect of intestinal microbiota on its recovery ability to repair damaged mucosal barrier. In this study, we established intestinal organoids and lamina propria lymphocytes (LPLs) co-cultured system, plus mice experiments, to explore the protective effect of Lactobacillus reuteri D8 on integrity of intestinal mucosa. We found that only live L. reuteri D8 was effective in protecting the morphology of intestinal organoids and normal proliferation of epithelial stained with EdU under TNF-α treatment, which was also further verified in mice experiments. L. reuteri D8 colonized in the intestinal mucosa and ameliorated intestinal mucosa damage caused by DSS treatment, including improvement of body weight, colon length, pathological change, and proliferation level VSports手机版. The repair process stimulated by L. reuteri D8 was also accompanied with increased numbers of Lgr5+ and lysozyme+ cells both in intestinal organoids and mice intestine. Furthermore, we demonstrated that D8 metabolite indole-3-aldehyde stimulated LPLs to secret IL-22 through aryl hydrocarbon receptor (AhR) and then induced phosphorylation of STAT3 to accelerate proliferation of intestinal epithelial, thus recovering damaged intestinal mucosa. Our findings indicate L. reuteri protects intestinal barrier and activates intestinal epithelial proliferation, which sheds light on treatment approaches for intestinal inflammation based on ISCs with probiotics Lactobacillus and daily probiotic consumption in heath foods. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures (V体育安卓版)


References
-
- Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. Stem cells in repair of gastrointestinal epithelia. Physiology. 2017;32:278–89. doi: 10.1152/physiol.00005.2017. - "V体育2025版" DOI - PMC - PubMed
Publication types
- Actions (V体育平台登录)
MeSH terms (VSports手机版)
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- Actions (V体育2025版)
- Actions (VSports在线直播)
- V体育ios版 - Actions
- Actions (VSports手机版)
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- VSports - Actions
- "V体育官网" Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- Actions (V体育官网入口)
Substances
V体育ios版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

