Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination
- PMID: 29352166
- PMCID: PMC5833833
- DOI: "VSports注册入口" 10.1038/s41419-017-0081-9
Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination
"V体育ios版" Abstract
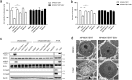
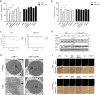
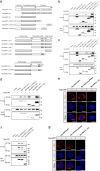
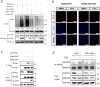
Necroptosis is an alternative programmed cell death pathway that is unleashed in the absence of apoptosis and mediated by signaling complexes containing receptor-interating protein kinase 1 (RIPK1) and RIPK3. This form of cell death has recently been implicated in host defense system to eliminate pathogen-infected cells VSports手机版. However, only a few viral species such as herpes simplex virus (HSV) and cytomegalovirus (CMV) have evolved mechanisms inhibiting necroptosis to overcome host antiviral defense, which is important for successful pathogenesis. Here, we show that the γ-herpesvirus Epstein-Barr virus (EBV) blocks necroptosis in EBV-infected human nasopharyngeal epithelial cells and nasopharyngeal carcinoma cells. Our findings indicate that EBV-encoded latent membrane protein 1 (LMP1), which lacks an RIP homotypic interaction motif (RHIM) domain, has mechanisms distinct from RHIM signaling competition to inhibit this necroptotic pathway. Intriguingly, LMP1 interacts directly with both RIPK1 and RIPK3 through its C-terminal activation region. More importantly, LMP1 can modulate the post-translational modification of the two receptor-interacting proteins. We then show that LMP1-mediated promotion of K63-polyubiquitinated RIPK1, suppression of RIPK1 protein expression and inhibition of K63-polyubiquitinated RIPK3 induced a switch in cell fate from necroptotic death to survival. These findings provide direct evidence for the suppression of necroptosis by EBV and define a mechanism of LMP1 to interrupt the initiation process of necroptosis before necrosome formation. .
Conflict of interest statement
All authors declare that they have no competing financial interests.
Figures


References (VSports手机版)
-
- Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. - DOI (VSports手机版) - PubMed
Publication types
- "VSports手机版" Actions
MeSH terms
- "V体育官网" Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- "V体育2025版" Actions
- Actions (V体育官网入口)
Substances
- "VSports注册入口" Actions
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育安卓版)
Molecular Biology Databases
"VSports" Research Materials
Miscellaneous

