Precision editing of the gut microbiota ameliorates colitis
- PMID: 29323293
- PMCID: PMC5804340
- DOI: 10.1038/nature25172
Precision editing of the gut microbiota ameliorates colitis
Abstract
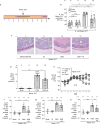
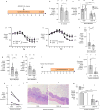
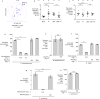
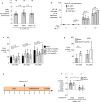
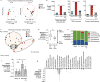
Inflammatory diseases of the gastrointestinal tract are frequently associated with dysbiosis, characterized by changes in gut microbial communities that include an expansion of facultative anaerobic bacteria of the Enterobacteriaceae family (phylum Proteobacteria). Here we show that a dysbiotic expansion of Enterobacteriaceae during gut inflammation could be prevented by tungstate treatment, which selectively inhibited molybdenum-cofactor-dependent microbial respiratory pathways that are operational only during episodes of inflammation VSports手机版. By contrast, we found that tungstate treatment caused minimal changes in the microbiota composition under homeostatic conditions. Notably, tungstate-mediated microbiota editing reduced the severity of intestinal inflammation in mouse models of colitis. We conclude that precision editing of the microbiota composition by tungstate treatment ameliorates the adverse effects of dysbiosis in the inflamed gut. .
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
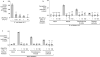


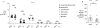


Comment in
-
Gut microbiota: Targeting of specific microbial species mitigates colitis.Nat Rev Gastroenterol Hepatol. 2018 Mar;15(3):132. doi: 10.1038/nrgastro.2018.4. Epub 2018 Jan 17. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29339809 No abstract available.
References
-
- Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. - PubMed (V体育安卓版)
Publication types
- "VSports手机版" Actions
MeSH terms
- "VSports手机版" Actions
- Actions (V体育2025版)
- V体育ios版 - Actions
- "VSports手机版" Actions
- VSports app下载 - Actions
- V体育2025版 - Actions
- "VSports app下载" Actions
- VSports在线直播 - Actions
- Actions (VSports)
- "V体育安卓版" Actions
- Actions (VSports在线直播)
- "V体育2025版" Actions
- "V体育安卓版" Actions
Substances
"V体育安卓版" Grants and funding
LinkOut - more resources (V体育平台登录)
"V体育官网入口" Full Text Sources
Other Literature Sources

