V体育官网入口 - Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4
- PMID: 29262324
- PMCID: PMC5746600
- DOI: 10.1016/j.celrep.2017.11.088
Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4
Abstract
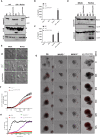
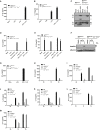
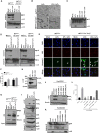
The caspase activation and recruitment domain (CARD)-based inflammasome sensors NLRP1b and NLRC4 induce caspase-1-dependent pyroptosis independent of the inflammasome adaptor ASC. Here, we show that NLRP1b and NLRC4 trigger caspase-8-mediated apoptosis as an alternative cell death program in caspase-1-/- macrophages and intestinal epithelial organoids (IECs). The caspase-8 adaptor FADD was recruited to ASC specks, which served as cytosolic platforms for caspase-8 activation and NLRP1b/NLRC4-induced apoptosis. We further found that caspase-1 protease activity dominated over scaffolding functions in suppressing caspase-8 activation and induction of apoptosis of macrophages and IECs. Moreover, TLR-induced c-FLIP expression inhibited caspase-8-mediated apoptosis downstream of ASC speck assembly, but did not affect pyroptosis induction by NLRP1b and NLRC4. Moreover, unlike during pyroptosis, NLRP1b- and NLRC4-elicited apoptosis retained alarmins and the inflammasome-matured cytokines interleukin 1β (IL-1β) and IL-18 intracellularly VSports手机版. This work identifies critical mechanisms regulating apoptosis induction by the inflammasome sensors NLRP1b and NLRC4 and suggests converting pyroptosis into apoptosis as a paradigm for suppressing inflammation. .
Keywords: ASC; NLRC4; NLRP1; apoptosis; caspase-1; caspase-8; cell death; inflammasome; inflammation; pyroptosis V体育安卓版. .
Copyright © 2017 The Author(s) V体育ios版. Published by Elsevier Inc. All rights reserved. .
"V体育官网" Figures

References
-
- Bossaller L., Chiang P.I., Schmidt-Lauber C., Ganesan S., Kaiser W.J., Rathinam V.A., Mocarski E.S., Subramanian D., Green D.R., Silverman N. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 2012;189:5508–5512. - PMC - PubMed
-
- Boyden E.D., Dietrich W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006;38:240–244. - "V体育安卓版" PubMed
-
- Bracaglia C., Gatto A., Pardeo M., Lapeyre G., Ferlin W., Nelson R., de Min C., De Benedetti F. Anti interferon-gamma (IFNγ) monoclonal antibody treatment in a patient carrying an NLRC4 mutation and severe hemophagocytic lymphohistiocytosis. Pediatric Rheumatology Online J. 2015;13:O68.
-
- Brydges S.D., Broderick L., McGeough M.D., Pena C.A., Mueller J.L., Hoffman H.M. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J. Clin. Invest. 2013;123:4695–4705. - "VSports" PMC - PubMed
MeSH terms
- Actions (V体育官网入口)
- Actions (V体育官网入口)
- Actions (VSports app下载)
- "VSports" Actions
- VSports最新版本 - Actions
Substances
- VSports - Actions
- Actions (VSports app下载)
- V体育ios版 - Actions
- "V体育ios版" Actions
- V体育官网入口 - Actions
- Actions (V体育平台登录)
"V体育ios版" Grants and funding
LinkOut - more resources
Full Text Sources (V体育官网)
Other Literature Sources (VSports app下载)
Molecular Biology Databases
V体育ios版 - Research Materials
Miscellaneous

