Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone
- PMID: 29237722
- PMCID: PMC5815904
- DOI: 10.1161/CIRCRESAHA.117.312380
"VSports" Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone
"VSports最新版本" Abstract
Rationale: Resistant hypertension is a major health concern with unknown cause. Spironolactone is an effective antihypertensive drug, especially for patients with resistant hypertension, and is considered by the World Health Organization as an essential medication VSports手机版. Although spironolactone can act at the mineralocorticoid receptor (MR; NR3C2), there is increasing evidence of MR-independent effects of spironolactone. .
Objective: Here, we detail the unexpected discovery that Panx1 (pannexin 1) channels could be a relevant in vivo target of spironolactone V体育安卓版. .
Methods and results: First, we identified spironolactone as a potent inhibitor of Panx1 in an unbiased small molecule screen, which was confirmed by electrophysiological analysis V体育ios版. Next, spironolactone inhibited α-adrenergic vasoconstriction in arterioles from mice and hypertensive humans, an effect dependent on smooth muscle Panx1, but independent of the MR NR3C2. Last, spironolactone acutely lowered blood pressure, which was dependent on smooth muscle cell expression of Panx1 and independent of NR3C2. This effect, however, was restricted to steroidal MR antagonists as a nonsteroidal MR antagonist failed to reduced blood pressure. .
Conclusions: These data suggest new therapeutic modalities for resistant hypertension based on Panx1 inhibition VSports最新版本. .
Keywords: hypertension; mice; mineralocorticoid; pannexin 1; spironolactone; vasoconstriction. V体育平台登录.
© 2017 American Heart Association, Inc.
Figures
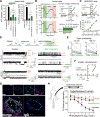
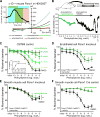
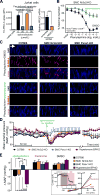

Comment in (V体育平台登录)
-
Pannexin Channel Inhibition: An Evolving Target to Lower Blood Pressure?Circ Res. 2018 Feb 16;122(4):543-545. doi: 10.1161/CIRCRESAHA.118.312566. Circ Res. 2018. PMID: 29449359 Free PMC article.
-
"VSports" Letter by Angus and Wright Regarding Article, "Pannexin-1 Channels as an Unexpected New Target of the Antihypertensive Drug Spironolactone".Circ Res. 2018 May 25;122(11):e86-e87. doi: 10.1161/CIRCRESAHA.118.313062. Circ Res. 2018. PMID: 29798904 No abstract available.
-
Response by Good et al to Letter Regarding Article, "Pannexin-1 Channels as an Unexpected New Target of the Antihypertensive Drug Spironolactone".Circ Res. 2018 May 25;122(11):e88-e89. doi: 10.1161/CIRCRESAHA.118.313080. Circ Res. 2018. PMID: 29798905 Free PMC article. No abstract available.
References
-
- Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. - PubMed
-
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. - "V体育平台登录" PMC - PubMed
-
- Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic p2×7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. - V体育ios版 - PMC - PubMed
-
- Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8:ra17. - PMC - PubMed
V体育官网入口 - Publication types
- VSports手机版 - Actions
V体育官网 - MeSH terms
- Actions (V体育平台登录)
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- "V体育2025版" Actions
- Actions (V体育官网)
- "VSports手机版" Actions
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- Actions (VSports手机版)
- VSports - Actions
- Actions (VSports)
Substances
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- Actions (V体育ios版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports app下载" Medical

