Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease
- PMID: 29211494
- PMCID: PMC6020409
- DOI: "V体育ios版" 10.1164/rccm.201704-0667OC
Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease
Abstract
Rationale: Up to 40% of smokers develop chronic obstructive pulmonary disease (COPD) over a period that spans decades. Despite the importance of COPD, much remains to be learned about susceptibility and pathogenesis, especially during early, prediagnostic stages of disease. Airway basal progenitor cells are crucial for lung health and resilience because of their ability to repair injured airways. In COPD, the normal airway epithelium is replaced with increased basal and secretory (mucous) cells and decreased ciliated cells, suggesting that progenitors are impaired. VSports手机版.
Objectives: To examine airway basal progenitor cells and lung function in smokers with and without COPD V体育安卓版. .
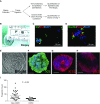
Methods: Bronchial biopsies taken from smokers at risk for COPD and lung cancer were used to acquire airway basal progenitor cells. They were evaluated for count, self-renewal, and multipotentiality (ability to differentiate to basal, mucous, and ciliated cells), and progenitor count was examined for its relationship with lung function. V体育ios版.
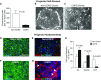
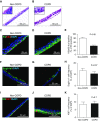
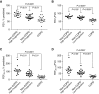
Measurements and main results: Basal progenitor count, self-renewal, and multipotentiality were all reduced in COPD versus non-COPD VSports最新版本. COPD progenitors produced an epithelium with increased basal and mucous cells and decreased ciliated cells, replicating the COPD phenotype. Progenitor depletion correlated with lung function and identified a subset of subjects without COPD with lung function that was midway between non-COPD with high progenitor counts and those with COPD. .
Conclusions: Basal progenitor dysfunction relates to the histologic and physiologic manifestations of COPD and identifies a subset that may represent an early, prediagnostic stage of COPD, indicating that progenitor exhaustion is involved in COPD pathogenesis V体育平台登录. .
Keywords: cell self-renewal; disease susceptibility; early diagnosis; multipotentiality; stem cells. VSports注册入口.
Figures (VSports最新版本)

Comment in
-
Airway Epithelial Progenitors and the Natural History of Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2018 Apr 1;197(7):847-849. doi: 10.1164/rccm.201711-2327ED. Am J Respir Crit Care Med. 2018. PMID: 29241015 Free PMC article. No abstract available.
-
Airway Basal Cell Reprogramming and Epithelial-Mesenchymal Transition: A Potential Key to Understanding Early Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2018 Jun 15;197(12):1644-1645. doi: 10.1164/rccm.201712-2450LE. Am J Respir Crit Care Med. 2018. PMID: 29385350 No abstract available.
-
"V体育ios版" Reply to Sohal: Airway Basal Cell Reprogramming and Epithelial-Mesenchymal Transition: A Potential Key to Understanding Early Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2018 Jun 15;197(12):1645-1646. doi: 10.1164/rccm.201801-0103LE. Am J Respir Crit Care Med. 2018. PMID: 29385351 Free PMC article. No abstract available.
References
-
- Fletcher CM, Peto R, Tinker C, Speizer RE. An eight-year study of early chronic obstructive lung disease in working men in London. London: Oxford University Press; 1976. The natural history of chronic bronchitis and emphysema.
-
- Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow Study. Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134:1011–1019. - "V体育平台登录" PubMed
-
- Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–1552. - PubMed
Publication types
MeSH terms
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- "VSports手机版" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
VSports最新版本 - Full Text Sources
Other Literature Sources
Medical

