Genomic and lipidomic analyses differentiate the compensatory roles of two COX isoforms during systemic inflammation in mice (V体育ios版)
- PMID: 29180443
- PMCID: PMC5748501
- DOI: 10.1194/jlr.M080028
Genomic and lipidomic analyses differentiate the compensatory roles of two COX isoforms during systemic inflammation in mice
V体育ios版 - Abstract
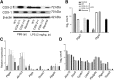
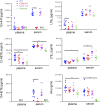
Both cyclooxygenase (COX)-1 and COX-2, encoded by Ptgs1 and Ptgs2, function coordinately during inflammation. But the relative contributions and compensations of COX-1 and COX-2 to inflammatory responses remain unanswered. We used three engineered mouse lines where the Ptgs1 and Ptgs2 genes substitute for one another to discriminate the distinct roles and interchangeability of COX isoforms during systemic inflammation. In macrophages, kidneys, and lungs, "flipped" Ptgs genes generate a "reversed" COX expression pattern, where the knock-in COX-2 is expressed constitutively and the knock-in COX-1 is lipopolysaccharide inducible. A panel of eicosanoids detected in serum and kidney demonstrates that prostaglandin (PG) biosynthesis requires native COX-1 and cannot be rescued by the knock-in COX-2. Our data further reveal preferential compensation of COX isoforms for prostanoid production in macrophages and throughout the body, as reflected by urinary PG metabolites. NanoString analysis indicates that inflammatory networks can be maintained by isoform substitution in inflamed macrophages. However, COX-1>COX-2 macrophages show reduced activation of inflammatory signaling pathways, indicating that COX-1 may be replaced by COX-2 within this complex milieu, but not vice versa. Collectively, each COX isoform plays a distinct role subject to subcellular environment and tissue/cell-specific conditions, leading to subtle compensatory differences during systemic inflammation VSports手机版. .
Keywords: animal model; cyclooxygenase; eicosanoid; lipopolysaccharide; macrophage; prostaglandin, gene targeting V体育安卓版. .
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc. V体育ios版.
Figures






References
-
- Smith W. L., DeWitt D. L., and Garavito R. M.. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69: 145–182. - "VSports最新版本" PubMed
-
- Simmons D. L., Botting R. M., and Hla T.. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56: 387–437. - PubMed (VSports app下载)
-
- Vane J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231: 232–235. - PubMed
Publication types
- VSports app下载 - Actions
MeSH terms
- "V体育ios版" Actions
- V体育ios版 - Actions
- Actions (VSports在线直播)
- VSports注册入口 - Actions
- "V体育安卓版" Actions
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- VSports最新版本 - Actions
V体育官网入口 - Substances
"VSports手机版" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports" Molecular Biology Databases
Research Materials

