Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma
- PMID: 29170503
- PMCID: PMC5701046
- DOI: 10.1038/s41467-017-01460-0
Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma
Erratum in
-
Author Correction: Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma.Nat Commun. 2020 Apr 1;11(1):1714. doi: 10.1038/s41467-020-15531-2. Nat Commun. 2020. PMID: 32238805 Free PMC article.
Abstract
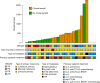
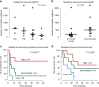
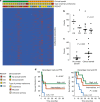
Adoptive T-cell therapy (ACT) is a highly intensive immunotherapy regime that has yielded remarkable response rates and many durable responses in clinical trials in melanoma; however, 50-60% of the patients have no clinical benefit. Here, we searched for predictive biomarkers to ACT in melanoma VSports手机版. Whole exome- and transcriptome sequencing and neoantigen prediction were applied to pre-treatment samples from 27 patients recruited to a clinical phase I/II trial of ACT in stage IV melanoma. All patients had previously progressed on other immunotherapies. We report that clinical benefit is associated with significantly higher predicted neoantigen load. High mutation and predicted neoantigen load are significantly associated with improved progression-free and overall survival. Further, clinical benefit is associated with the expression of immune activation signatures including a high MHC-I antigen processing and presentation score. These results improve our understanding of mechanisms behind clinical benefit of ACT in melanoma. .
Conflict of interest statement
The authors declare no competing financial interests.
VSports注册入口 - Figures


VSports在线直播 - References
-
- Svane IM, Verdegaal EM. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care? Cancer Immunol. Immunother. 2014;63:1081–1091. - VSports - PMC - PubMed
-
- Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. - PMC (VSports在线直播) - PubMed
-
- Besser MJ, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. 2013;19:4792–4800. - PubMed
-
- Besser MJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 2010;16:2646–2655. - PubMed
"VSports手机版" Publication types
MeSH terms
- VSports app下载 - Actions
- VSports手机版 - Actions
- V体育ios版 - Actions
- Actions (V体育ios版)
- Actions (V体育官网)
- V体育安卓版 - Actions
- VSports - Actions
- "VSports最新版本" Actions
- VSports - Actions
"V体育官网" Substances
- "VSports" Actions
"V体育平台登录" LinkOut - more resources
Full Text Sources (VSports手机版)
Other Literature Sources
Medical
Molecular Biology Databases
V体育官网入口 - Research Materials

