Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression
- PMID: 29157665
- PMCID: PMC5718096
- DOI: 10.1016/j.ajpath.2017.08.005
Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression
Abstract
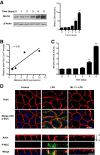
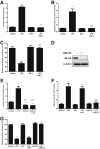
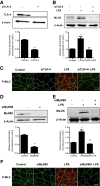
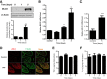
Lipopolysaccharides (LPSs) are a major component of the Gram-negative bacterial cell wall and play an important role in mediating intestinal inflammatory responses in inflammatory bowel disease. Although recent studies suggested that physiologically relevant concentrations of LPS (0 to 1 ng/mL) cause an increase in intestinal epithelial tight junction (TJ) permeability, the mechanisms that mediate an LPS-induced increase in intestinal TJ permeability remain unclear. Herein, we show that myosin light chain kinase (MLCK) plays a central role in the LPS-induced increase in TJ permeability VSports手机版. Filter-grown Caco-2 intestinal epithelial monolayers and C57BL/6 mice were used as an in vitro and in vivo intestinal epithelial model system, respectively. LPS caused a dose- and time-dependent increase in MLCK expression and kinase activity in Caco-2 monolayers. The pharmacologic MLCK inhibition and siRNA-induced knock-down of MLCK inhibited the LPS-induced increase in Caco-2 TJ permeability. The LPS increase in TJ permeability was mediated by toll-like receptor 4 (TLR-4)/MyD88 signal-transduction pathway up-regulation of MLCK expression. The LPS-induced increase in mouse intestinal permeability also required an increase in MLCK expression. The LPS-induced increase in intestinal permeability was inhibited in MLCK-/- and TLR-4-/- mice. These data show, for the first time, that the LPS-induced increase in intestinal permeability was mediated by TLR-4/MyD88 signal-transduction pathway up-regulation of MLCK. Therapeutic targeting of these pathways can prevent an LPS-induced increase in intestinal permeability. .
Published by Elsevier Inc.
Figures
References
-
- Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. - PMC (V体育平台登录) - PubMed
-
- Caradonna L., Amati L., Magrone T., Pellegrino N.M., Jirillo E., Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. - PubMed
-
- Scaldaferri F., Lopetuso L.R., Petito V., Cufino V., Bilotta M., Arena V., Stigliano E., Maulucci G., Papi M., Emiliana C.M., Poscia A., Franceschi F., Delogu G., Sanguinetti M., Spirito M.D., Sgambato A., Gasbarrini A. Gelatin tannate ameliorates acute colitis in mice by reinforcing mucus layer and modulating gut microbiota composition: emerging role for “gut barrier protectors” in IBD? United European Gastroenterol J. 2014;2:113–122. - PMC - PubMed
"VSports注册入口" MeSH terms
- "V体育ios版" Actions
- "VSports手机版" Actions
- Actions (VSports app下载)
- Actions (V体育官网)
- Actions (V体育2025版)
- "VSports手机版" Actions
- "V体育安卓版" Actions
- V体育ios版 - Actions
Substances
- V体育安卓版 - Actions
- "VSports" Actions
- "VSports注册入口" Actions
Grants and funding (VSports)
LinkOut - more resources
Full Text Sources (VSports app下载)
Other Literature Sources
Molecular Biology Databases

