Human resistin protects against endotoxic shock by blocking LPS-TLR4 interaction
- PMID: 29133417
- PMCID: "V体育平台登录" PMC5715788
- DOI: V体育平台登录 - 10.1073/pnas.1716015114
V体育2025版 - Human resistin protects against endotoxic shock by blocking LPS-TLR4 interaction
Abstract
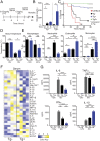
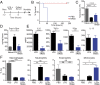
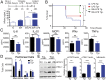
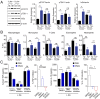
Helminths trigger multiple immunomodulatory pathways that can protect from sepsis. Human resistin (hRetn) is an immune cell-derived protein that is highly elevated in helminth infection and sepsis. However, the function of hRetn in sepsis, or whether hRetn influences helminth protection against sepsis, is unknown. Employing hRetn-expressing transgenic mice (hRETNTg+) and recombinant hRetn, we identify a therapeutic function for hRetn in lipopolysaccharide (LPS)-induced septic shock. hRetn promoted helminth-induced immunomodulation, with increased survival of Nippostrongylus brasiliensis (Nb)-infected hRETNTg+ mice after a fatal LPS dose compared with naive mice or Nb-infected hRETNTg- mice VSports手机版. Employing immunoprecipitation assays, hRETNTg+Tlr4-/- mice, and human immune cell culture, we demonstrate that hRetn binds the LPS receptor Toll-like receptor 4 (TLR4) through its N terminal and modulates STAT3 and TBK1 signaling, triggering a switch from proinflammatory to anti-inflammatory responses. Further, we generate hRetn N-terminal peptides that are able to block LPS proinflammatory function. Together, our studies identify a critical role for hRetn in blocking LPS function with important clinical significance in helminth-induced immunomodulation and sepsis. .
Keywords: LPS; TLR4; inflammation; resistin; sepsis. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Angus DC, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Cauwels A, Brouckaert P. Survival of TNF toxicity: Dependence on caspases and NO. Arch Biochem Biophys. 2007;462:132–139. - PubMed
-
- Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203. - PubMed
-
- Szkudlapski D, et al. The emering role of helminths in treatment of the inflammatory bowel disorders. J Physiol Pharmacol. 2014;65:741–751. - PubMed
Publication types
MeSH terms
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- "V体育2025版" Actions
- Actions (V体育2025版)
- V体育官网入口 - Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
- "VSports" Actions
- Actions (VSports app下载)
- "V体育ios版" Actions
- "VSports app下载" Actions
- "VSports" Actions
Substances
- "VSports" Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
- Actions (VSports注册入口)
Grants and funding
V体育官网 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

