Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes
- PMID: 29127238
- PMCID: PMC5899260 (VSports注册入口)
- DOI: 10.1152/ajpheart.00452.2017
Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes
Abstract
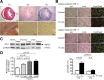
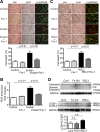
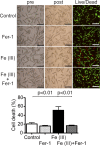
Clinical studies have suggested that myocardial iron is a risk factor for left ventricular remodeling in patients after myocardial infarction. Ferroptosis has recently been reported as a mechanism of iron-dependent nonapoptotic cell death. However, ferroptosis in the heart is not well understood. Mechanistic target of rapamycin (mTOR) protects the heart against pathological stimuli such as ischemia. To define the role of cardiac mTOR on cell survival in iron-mediated cell death, we examined cardiomyocyte (CM) cell viability under excess iron and ferroptosis conditions. Adult mouse CMs were isolated from cardiac-specific mTOR transgenic mice, cardiac-specific mTOR knockout mice, or control mice. CMs were treated with ferric iron [Fe(III)]-citrate, erastin, a class 1 ferroptosis inducer, or Ras-selective lethal 3 (RSL3), a class 2 ferroptosis inducer. Live/dead cell viability assays revealed that Fe(III)-citrate, erastin, and RSL3 induced cell death. Cotreatment with ferrostatin-1, a ferroptosis inhibitor, inhibited cell death in all conditions. mTOR overexpression suppressed Fe(III)-citrate, erastin, and RSL3-induced cell death, whereas mTOR deletion exaggerated cell death in these conditions. 2',7'-Dichlorodihydrofluorescein diacetate measurement of reactive oxygen species (ROS) production showed that erastin-induced ROS production was significantly lower in mTOR transgenic versus control CMs. These findings suggest that ferroptosis is a significant type of cell death in CMs and that mTOR plays an important role in protecting CMs against excess iron and ferroptosis, at least in part, by regulating ROS production VSports手机版. Understanding the effects of mTOR in preventing iron-mediated cell death will provide a new therapy for patients with myocardial infarction. NEW & NOTEWORTHY Ferroptosis has recently been reported as a new form of iron-dependent nonapoptotic cell death. However, ferroptosis in the heart is not well characterized. Using cultured adult mouse cardiomyocytes, we demonstrated that the mechanistic target of rapamycin plays an important role in protecting cardiomyocytes against excess iron and ferroptosis. .
Keywords: cardiomyocyte; ferroptosis; iron; mechanistic target of rapamycin V体育安卓版. .
Figures



Comment in
-
Ferroptosis: beating on death's door.Am J Physiol Heart Circ Physiol. 2018 Apr 1;314(4):H772-H775. doi: 10.1152/ajpheart.00692.2017. Epub 2017 Dec 6. Am J Physiol Heart Circ Physiol. 2018. PMID: 29212794 No abstract available.
References
-
- Aoyagi T, Higa JK, Aoyagi H, Yorichika N, Shimada BK, Matsui T. Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 308: H1530–H1539, 2015. doi:10.1152/ajpheart.00008.2015. - "VSports手机版" DOI - PMC - PubMed
-
- Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol 221: 117–124, 2010. doi:10.1002/path.2694. - DOI (V体育安卓版) - PMC - PubMed
Publication types
- Actions (VSports app下载)
- Actions (VSports注册入口)
"VSports" MeSH terms
- V体育官网 - Actions
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- V体育官网入口 - Actions
Substances
- "V体育ios版" Actions
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources (V体育官网入口)
Full Text Sources (VSports最新版本)
Other Literature Sources (VSports app下载)
VSports手机版 - Medical
Molecular Biology Databases
Miscellaneous

