Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells
- PMID: 29109552
- PMCID: PMC5673935
- DOI: 10.1038/s41598-017-15099-w
Butyrate and propionate inhibit antigen-specific CD8VSports在线直播 - + T cell activation by suppressing IL-12 production by antigen-presenting cells
Abstract
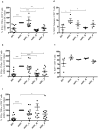
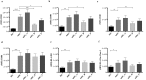
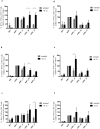
Short chain fatty acids (SCFAs), such as acetate, butyrate and propionate, are products of microbial macronutrients fermentation that distribute systemically and are believed to modulate host immune responses VSports手机版. Recent data have indicated that certain SCFAs, such as butyrate and propionate, directly modulate human dendritic cell (DC) function. Given the role of DCs in initiating and shaping the adaptive immune response, we now explore how SCFAs affect the activation of antigen-specific CD8+ T cells stimulated with autologous, MART1 peptide-pulsed DC. We show that butyrate reduces the frequency of peptide-specific CD8+ T cells and, together with propionate, inhibit the activity of those cells. On the contrary, acetate does not affect them. Importantly, butyrate and propionate inhibit the production of IL-12 and IL-23 in the DCs and exogenous IL-12 fully restores the activation of the MART-1-specific CD8+ T cells, whereas IL-23 has no effect. In conclusion, these results point to a pivotal role of butyrate and propionate in modulating CD8+ T cell activation via the inhibition of IL-12 secretion from DCs. These findings reveal a novel mechanism whereby bacterial fermentation products may modulate CD8+ T cell function with possible implications in anti-cancer immunotherapy. .
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Krautkramer KA, et al. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol Cell. 2016;64:982–992. doi: 10.1016/j.molcel.2016.10.025. - "VSports手机版" DOI - PMC - PubMed
MeSH terms
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- Actions (V体育官网)
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- "VSports最新版本" Actions
Substances (V体育平台登录)
- "VSports手机版" Actions
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

