"VSports手机版" Extrafollicular CD4+ T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease
- PMID: 29042531
- PMCID: PMC5645449 (VSports在线直播)
- DOI: 10.1038/s41467-017-00880-2
Extrafollicular CD4+ T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease
VSports app下载 - Abstract
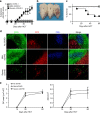
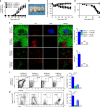
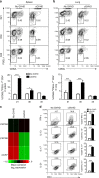
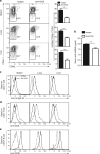
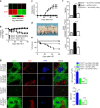
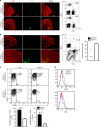
Chronic graft-versus-host disease (cGVHD) is an autoimmune-like syndrome mediated by pathogenic CD4+ T and B cells, but the function of extrafollicular and germinal center CD4+ T and B interactions in cGVHD pathogenesis remains largely unknown. Here we show that extrafollicular CD4+ T and B interactions are sufficient for inducing cGVHD, while germinal center formation is dispensable. The pathogenesis of cGVHD is associated with the expansion of extrafollicular CD44hiCD62loPSGL-1loCD4+ (PSGL-1loCD4+) T cells. These cells express high levels of ICOS, and the blockade of ICOS/ICOSL interaction prevents their expansion and ameliorates cGVHD. Expansion of PSGL-1loCD4+ T cells is also prevented by BCL6 or Stat3 deficiency in donor CD4+ T cells, with the induction of cGVHD ameliorated by BCL6 deficiency and completely suppressed by Stat3 deficiency in donor CD4+ T cells. These results support that Stat3- and BCL6-dependent extrafollicular CD4+ T and B interactions play critical functions in the pathogenesis of cGVHD. Chronic graft-versus-host disease (cGVHD) is mediated by specific CD4 and B cells, but the relative contribution of extrafollicular and germinal centre (GC) T-B interaction is unclear. Here the authors show that the extrafollicular expansion of a specific CD4 T subset is sufficient for inducing cGVHD while GC is dispensable. VSports手机版.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


"V体育安卓版" References
-
- M. Horowitz, M. in Thomas’ Hematopoietic Cell Transplantation 5th edn (eds Forman, S. J., Negrin, R. S., Antin J. H. & Appelbaum F. R.) (Wiley-Blackwell, 2016).
Publication types
- Actions (V体育平台登录)
MeSH terms
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- V体育安卓版 - Actions
- V体育安卓版 - Actions
- Actions (VSports)
- V体育ios版 - Actions
- Actions (V体育官网)
- "V体育2025版" Actions
- Actions (V体育2025版)
- V体育安卓版 - Actions
Substances
- VSports app下载 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

