Induction of Apoptosis and Subsequent Phagocytosis of Virus-Infected Cells As an Antiviral Mechanism
- PMID: 29033939
- PMCID: PMC5624992
- DOI: 10.3389/fimmu.2017.01220
"VSports手机版" Induction of Apoptosis and Subsequent Phagocytosis of Virus-Infected Cells As an Antiviral Mechanism
Abstract
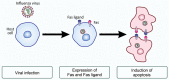
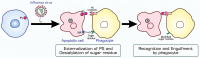
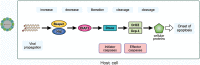
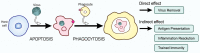
Viruses are infectious entities that hijack host replication machineries to produce their progeny, resulting, in most cases, in disease and, sometimes, in death in infected host organisms. Hosts are equipped with an array of defense mechanisms that span from innate to adaptive as well as from humoral to cellular immune responses. We previously demonstrated that mouse cells underwent apoptosis in response to influenza virus infection VSports手机版. These apoptotic, virus-infected cells were then targeted for engulfment by macrophages and neutrophils. We more recently reported similar findings in the fruit fly Drosophila melanogaster, which lacks adaptive immunity, after an infection with Drosophila C virus. In these experiments, the inhibition of phagocytosis led to severe influenza pathologies in mice and early death in Drosophila. Therefore, the induction of apoptosis and subsequent phagocytosis of virus-infected cells appear to be an antiviral innate immune mechanism that is conserved among multicellular organisms. We herein discuss the underlying mechanisms and significance of the apoptosis-dependent phagocytosis of virus-infected cells. Investigations on the molecular and cellular features responsible for this underrepresented virus-host interaction may provide a promising avenue for the discovery of novel substances that are targeted in medical treatments against virus-induced intractable diseases. .
Keywords: antiviral mechanism; apoptosis; innate immunity; phagocytosis; viral infection. V体育安卓版.
Figures
References
-
- Lindahl JF, Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol (2015) 5:30048. 10.3402/iee.v5.30048 - DOI (VSports注册入口) - PMC - PubMed
Publication types
- "VSports app下载" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Molecular Biology Databases
Research Materials (V体育官网入口)

