Building the CuA site of cytochrome c oxidase: A complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins
- PMID: 28972150
- PMCID: PMC5880131
- DOI: 10.1074/jbc.R117.816132
"V体育平台登录" Building the CuA site of cytochrome c oxidase: A complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins
Abstract
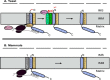
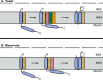
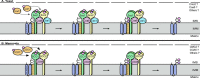
Cytochrome c oxidase (COX) was initially purified more than 70 years ago. A tremendous amount of insight into its structure and function has since been gleaned from biochemical, biophysical, genetic, and molecular studies. As a result, we now appreciate that COX relies on its redox-active metal centers (heme a and a3, CuA and CuB) to reduce oxygen and pump protons in a reaction essential for most eukaryotic life. Questions persist, however, about how individual structural subunits are assembled into a functional holoenzyme. Here, we focus on what is known and what remains to be learned about the accessory proteins that facilitate CuA site maturation VSports手机版. .
Keywords: COX assembly factors; CuA site formation; chaperone; copper; cytochrome c oxidase (complex IV); mitochondria; protein assembly. V体育安卓版.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc. V体育ios版.
"V体育官网" Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
V体育官网入口 - References
Publication types
- "V体育平台登录" Actions
MeSH terms
- "V体育官网" Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- V体育官网入口 - Actions
- V体育安卓版 - Actions
"V体育官网入口" Substances
- Actions (V体育平台登录)
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports手机版" Research Materials

