"VSports在线直播" Bortezomib resistance in multiple myeloma is associated with increased serine synthesis
- PMID: 28855983
- PMCID: PMC5575874
- DOI: "VSports" 10.1186/s40170-017-0169-9
Bortezomib resistance in multiple myeloma is associated with increased serine synthesis
Abstract
Background: The proteasome inhibitor bortezomib (BTZ) is successfully applied in the treatment of multiple myeloma, but its efficacy is restricted by the wide-spread occurrence of resistance VSports手机版. Metabolic alterations play an important role in cancer development and aid in the cellular adaptation to pharmacologically changed environments. Metabolic changes could therefore play an essential role in the development of drug resistance. However, specific metabolic pathways that can be targeted to improve bortezomib therapy remain unidentified. .
Methods: We elucidated the metabolic mechanisms underlying bortezomib resistance by using mass spectrometry-based metabolomics and proteomics on BTZ-sensitive and BTZ-resistant multiple myeloma cell lines as well as in a set of CD138+ cells obtained from multiple myeloma patients V体育安卓版. .
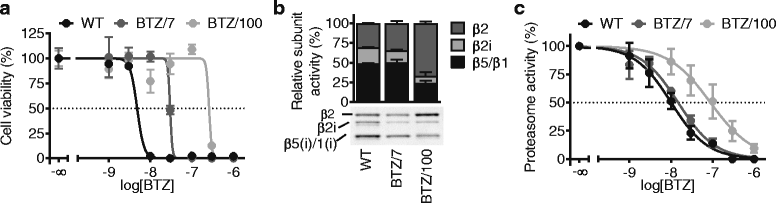
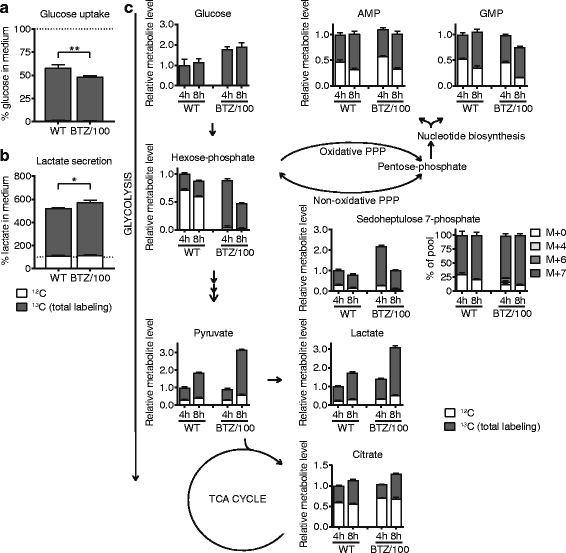
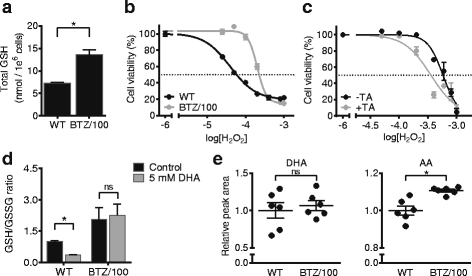
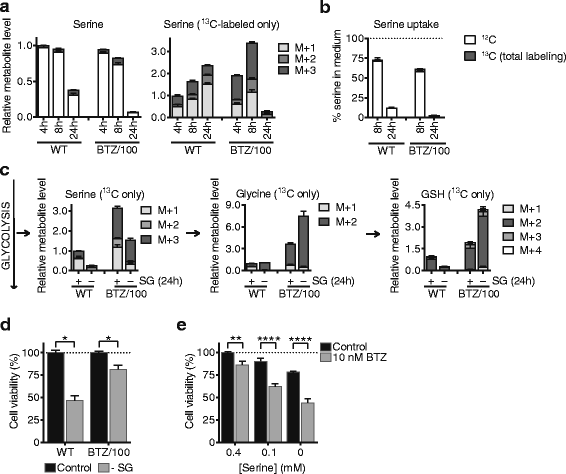
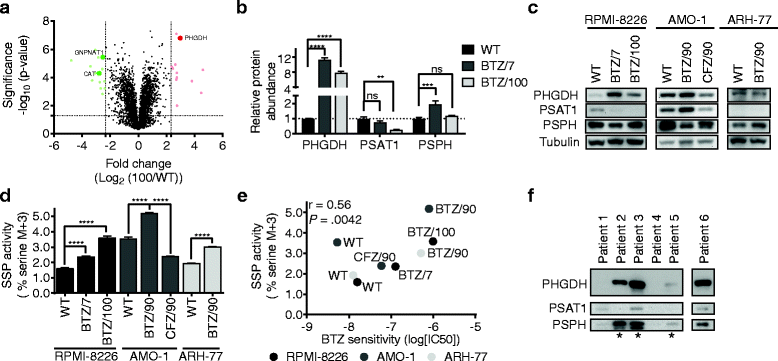
Results: Our findings demonstrate that a rewired glucose metabolism sustains bortezomib resistance V体育ios版. Mechanistically, this results in higher activity of both the pentose phosphate pathway and serine synthesis pathway, ultimately leading to an increased anti-oxidant capacity of BTZ-resistant cells. Moreover, our results link both serine synthesis pathway activity and expression of 3-phosphoglycerate dehydrogenase (PHGDH), which catalyzes the rate-limiting step of serine synthesis, to bortezomib resistance across different BTZ-resistant multiple myeloma cell lines. Consistently, serine starvation enhanced the cytotoxicity of bortezomib, underscoring the importance of serine metabolism in the response to BTZ. Importantly, in CD138+ cells of clinically bortezomib refractory multiple myeloma patients, PHGDH expression was also markedly increased. .
Conclusions: Our findings indicate that interfering with serine metabolism may be a novel strategy to improve bortezomib therapy and identify PHGDH as a potential biomarker for BTZ resistance. VSports最新版本.
Keywords: Bortezomib; Drug resistance; Metabolism; Multiple myeloma; PHGDH V体育平台登录. .
Conflict of interest statement
Ethics approval and consent to participate
Research was approved by the Medical Ethics Committee of the VU University Medical Center and all patients gave written informed consent.
Consent for publication
Not applicable
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
"VSports最新版本" Figures
References
-
- Raynes R, Pomatto LCD, Davies KJA. Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol Aspects Med. 2016;50:41–55. - "VSports app下载" PMC - PubMed
LinkOut - more resources
"V体育平台登录" Full Text Sources
Other Literature Sources
Miscellaneous (VSports在线直播)

