Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells
- PMID: 28830878
- PMCID: PMC5669830
- DOI: "V体育官网" 10.1158/2159-8290.CD-17-0538
Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells
VSports手机版 - Abstract
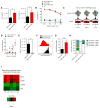
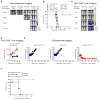
Successful adoptive T-cell immunotherapy of solid tumors will require improved expansion and cytotoxicity of tumor-directed T cells within tumors. Providing recombinant or transgenic cytokines may produce the desired benefits but is associated with significant toxicities, constraining clinical use. To circumvent this limitation, we constructed a constitutively signaling cytokine receptor, C7R, which potently triggers the IL7 signaling axis but is unresponsive to extracellular cytokine. This strategy augments modified T-cell function following antigen exposure, but avoids stimulating bystander lymphocytes. Coexpressing the C7R with a tumor-directed chimeric antigen receptor (CAR) increased T-cell proliferation, survival, and antitumor activity during repeated exposure to tumor cells, without T-cell dysfunction or autonomous T-cell growth. Furthermore, C7R-coexpressing CAR T cells were active against metastatic neuroblastoma and orthotopic glioblastoma xenograft models even at cell doses that had been ineffective without C7R support. C7R may thus be able to enhance antigen-specific T-cell therapies against cancer. Significance: The constitutively signaling C7R system developed here delivers potent IL7 stimulation to CAR T cells, increasing their persistence and antitumor activity against multiple preclinical tumor models, supporting its clinical development. Cancer Discov; 7(11); 1238-47. ©2017 AACR. This article is highlighted in the In This Issue feature, p VSports手机版. 1201. .
©2017 American Association for Cancer Research. V体育安卓版.
Conflict of interest statement
Figures


References
-
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer Nature Publishing Group. 2013;13:525–41. - PubMed
-
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. - "V体育官网入口" PMC - PubMed
MeSH terms
- Actions (V体育2025版)
- VSports app下载 - Actions
- "VSports app下载" Actions
- V体育官网入口 - Actions
- "VSports在线直播" Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
Substances
- "VSports注册入口" Actions
Grants and funding (VSports在线直播)
"VSports app下载" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (V体育安卓版)

