"VSports最新版本" HLA-Bw4-I-80 Isoform Differentially Influences Clinical Outcome As Compared to HLA-Bw4-T-80 and HLA-A-Bw4 Isoforms in Rituximab or Dinutuximab-Based Cancer Immunotherapy
- PMID: 28659916
- PMCID: PMC5466980
- DOI: 10.3389/fimmu.2017.00675
HLA-Bw4-I-80 Isoform Differentially Influences Clinical Outcome As Compared to HLA-Bw4-T-80 and HLA-A-Bw4 Isoforms in Rituximab or Dinutuximab-Based Cancer Immunotherapy
Abstract (VSports手机版)
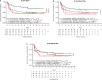
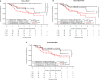
Killer-cell immunoglobulin-like receptors (KIRs) are a family of glycoproteins expressed primarily on natural killer cells that can regulate their function. Inhibitory KIRs recognize MHC class I molecules (KIR-ligands) as ligands. We have reported associations of KIRs and KIR-ligands for patients in two monoclonal antibody (mAb)-based trials: (1) A Children's Oncology Group (COG) trial for children with high-risk neuroblastoma randomized to immunotherapy treatment with dinutuximab (anti-GD2 mAb) + GM-CSF + IL-2 + isotretinion or to treatment with isotretinoin alone and (2) An Eastern Cooperative Oncology Group (ECOG) trial for adults with low-tumor burden follicular lymphoma responding to an induction course of rituximab (anti-CD20 mAb) and randomized to treatment with maintenance rituximab or no-maintenance rituximab. In each trial, certain KIR/KIR-ligand genotypes were associated with clinical benefit for patients randomized to immunotherapy treatment (immunotherapy in COG; maintenance rituximab in ECOG) as compared to patients that did not receive the immunotherapy [isotretinoin alone (COG); no-maintenance (ECOG)]. Namely, patients with both KIR3DL1 and its HLA-Bw4 ligand (KIR3DL1+/HLA-Bw4+ genotype) had improved clinical outcomes if randomized to immunotherapy regimens, as compared to patients with the KIR3DL1+/HLA-Bw4+ genotype randomized to the non-immunotherapy regimen. Conversely, patients that did not have the KIR3DL1+/HLA-Bw4+ genotype showed no evidence of a difference in outcome if receiving the immunotherapy vs VSports手机版. no-immunotherapy. For each trial, HLA-Bw4 status was determined by assessing the genotypes of three separate isoforms of HLA-Bw4: (1) HLA-B-Bw4 with threonine at amino acid 80 (B-Bw4-T80); (2) HLA-B-Bw4 with isoleucine at amino acid 80 (HLA-B-Bw4-I80); and (3) HLA-A with a Bw4 epitope (HLA-A-Bw4). Here, we report on associations with clinical outcome for patients with KIR3DL1 and these separate isoforms of HLA-Bw4. Patients randomized to immunotherapy with KIR3DL1+/A-Bw4+ or with KIR3DL1+/B-Bw4-T80+ had better outcome vs. those randomized to no-immunotherapy, whereas for those with KIR3DL1+/B-Bw4-I80+ there was no evidence of a difference based on immunotherapy vs. no-immunotherapy. Additionally, we observed differences within treatment types (either within immunotherapy or no-immunotherapy) that were associated with the genotype status for the different KIR3DL1/HLA-Bw4-isoforms. These studies suggest that specific HLA-Bw4 isoforms may differentially influence response to these mAb-based immunotherapy, further confirming the involvement of KIR-bearing cells in tumor-reactive mAb-based cancer immunotherapy. .
Keywords: HLA; HLA-Bw4; KIR; KIR-ligand; MHC class I; cancer immunotherapy; natural killer cells. V体育安卓版.
Figures
References
-
- Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol Immunol (2002) 38(14):1007–21.10.1016/S0161-5890(02)00030-5 - V体育ios版 - DOI - PubMed
Grants and funding
VSports注册入口 - LinkOut - more resources
Full Text Sources (V体育官网入口)
Other Literature Sources
"VSports注册入口" Research Materials

