Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site
- PMID: 28533431
- PMCID: PMC5519355
- DOI: 10.1074/jbc.M117.775981
Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site
Abstract
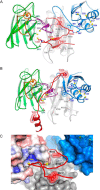
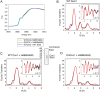
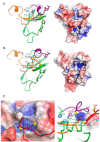
Metallochaperones are a diverse family of trafficking molecules that provide metal ions to protein targets for use as cofactors. The copper chaperone for superoxide dismutase (Ccs1) activates immature copper-zinc superoxide dismutase (Sod1) by delivering copper and facilitating the oxidation of the Sod1 intramolecular disulfide bond. Here, we present structural, spectroscopic, and cell-based data supporting a novel copper-induced mechanism for Sod1 activation. Ccs1 binding exposes an electropositive cavity and proposed "entry site" for copper ion delivery on immature Sod1. Copper-mediated sulfenylation leads to a sulfenic acid intermediate that eventually resolves to form the Sod1 disulfide bond with concomitant release of copper into the Sod1 active site. Sod1 is the predominant disulfide bond-requiring enzyme in the cytoplasm, and this copper-induced mechanism of disulfide bond formation obviates the need for a thiol/disulfide oxidoreductase in that compartment VSports手机版. .
Keywords: X-ray crystallography; chaperone; copper; enzyme activation; metalloenzyme; superoxide dismutase (SOD). V体育安卓版.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc V体育ios版. .
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
VSports app下载 - Figures





"VSports在线直播" References
-
- Culotta V. C., Klomp L. W., Strain J., Casareno R. L., Krems B., and Gitlin J. D. (1997) The copper chaperone for superoxide dismutase. J. Biol. Chem. 272, 23469–23472 - PubMed
-
- Fridovich I. (1989) Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 264, 7761–7764 - PubMed
-
- Arnesano F., Banci L., Bertini I., Martinelli M., Furukawa Y., and O'Halloran T. V. (2004) The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J. Biol. Chem. 279, 47998–48003 - PubMed (VSports app下载)
-
- Doucette P. A., Whitson L. J., Cao X., Schirf V., Demeler B., Valentine J. S., Hansen J. C., and Hart P. J. (2004) Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J. Biol. Chem. 279, 54558–54566 - PubMed
Publication types
- "V体育2025版" Actions
- "VSports最新版本" Actions
MeSH terms
- V体育2025版 - Actions
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- "V体育2025版" Actions
- "VSports注册入口" Actions
- Actions (VSports)
- Actions (V体育安卓版)
- "V体育平台登录" Actions
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- "V体育官网入口" Actions
- VSports最新版本 - Actions
- "VSports手机版" Actions
- "VSports注册入口" Actions
- Actions (VSports手机版)
Substances
- VSports app下载 - Actions
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- Actions (VSports注册入口)
- Actions (VSports app下载)
- VSports注册入口 - Actions
"VSports手机版" Associated data
- "VSports在线直播" Actions
- Actions
- V体育平台登录 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

