MYCN induces neuroblastoma in primary neural crest cells (VSports)
- PMID: 28459463
- PMCID: PMC5582212
- DOI: 10.1038/onc.2017.128
MYCN induces neuroblastoma in primary neural crest cells
Abstract
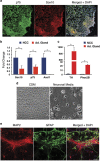
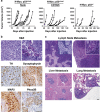
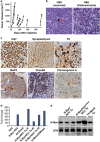
Neuroblastoma (NBL) is an embryonal cancer of the sympathetic nervous system (SNS), which causes 15% of pediatric cancer deaths. High-risk NBL is characterized by N-Myc amplification and segmental chromosomal gains and losses. Owing to limited disease models, the etiology of NBL is largely unknown, including both the cell of origin and the majority of oncogenic drivers. We have established a novel system for studying NBL based on the transformation of neural crest cells (NCCs), the progenitor cells of the SNS, isolated from mouse embryonic day 9. 5 trunk neural tube explants. Based on pathology and gene expression analysis, we report the first successful transformation of wild-type NCCs into NBL by enforced expression of N-Myc, to generate phenotypically and molecularly accurate tumors that closely model human MYCN-amplified NBL. Using comparative genomic hybridization, we found that NCC-derived NBL tumors acquired copy number gains and losses that are syntenic to those observed in human MYCN-amplified NBL including 17q gain, 2p gain and loss of 1p36 VSports手机版. When p53-compromised NCCs were transformed with N-Myc, we generated primitive neuroectodermal tumors with divergent differentiation including osteosarcoma. These subcutaneous tumors were metastatic to regional lymph nodes, liver and lung. Our novel experimental approach accurately models human NBL and establishes a new system with potential to study early stages of NBL oncogenesis, to functionally assess NBL oncogenic drivers and to characterize NBL metastasis. .
"V体育2025版" Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Meany HJ, London WB, Ambros PF, Matthay KK, Monclair T, Simon T et al. Significance of clinical and biologic features in Stage 3 neuroblastoma: a report from the International Neuroblastoma Risk Group project. Pediatr Blood Cancer 2014; 61: 1932–1939. - "V体育官网入口" PubMed
Publication types
MeSH terms
- "V体育官网" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- "V体育官网" Actions
- Actions (V体育ios版)
- "VSports手机版" Actions
- "V体育安卓版" Actions
- Actions (VSports手机版)
- Actions (VSports)
- V体育官网 - Actions
- "VSports注册入口" Actions
Substances
- "VSports注册入口" Actions
- Actions (V体育平台登录)
V体育2025版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
"VSports" Research Materials
Miscellaneous

