VSports app下载 - TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis
- PMID: 28358372
- PMCID: PMC5386532
- DOI: VSports注册入口 - 10.1038/cddis.2017.129
TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis
Abstract
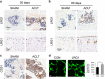
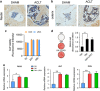
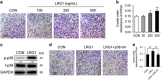
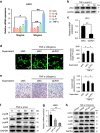
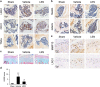
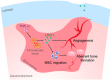
The incomplete understanding of aberrant neovascularization, which contributes to osteoarthritis suggests that additional modulators have yet to be identified. Our objective was to identify the role of Leucine-rich-alpha-2-glycoprotein1 (LRG1), a new regulator of pathogenic angiogenesis, in osteoarthritis progression and to develop effective treatment strategies. In this study, immunohistochemistry showed that LRG1 was increased in the subchondral bone and articular cartilage in anterior cruciate ligament transection (ACLT) mice. Further studies were focused on the role of LRG1 in osteoarthritis. Results showed that LRG1 promoted angiogenesis and mesenchymal stem cells (MSC) migration, which contribute to aberrant bone formation in the subchondral bone. Moreover, tumor necrosis factor-α (TNF-α), not interleukin-1β (IL-1β), IL-6 or IL-17, induced the LRG1 expression in human umbilical vein endothelial cells and this effect was inhibited by p38 mitogen-activated protein kinase or NF-κB inhibitor. Notably, inhibition of TNF-α and LRG1 activity by Lenalidomide, an inhibitor of TNF-α production, in ACLT mice attenuated degeneration of osteoarthritis articular cartilage. This study shows that TNF-α is the predominant proinflammatory cytokine that induces the secretion of LRG1. LRG1 contributes to angiogenesis-coupled de novo bone formation by increasing angiogenesis and recruiting MSCs in the subchondral bone of osteoarthritis joints. Inhibition of TNF-α and LRG1 by Lenalidomide could be a potential therapeutic approach VSports手机版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 2006; 54: 226–229. - PubMed
-
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011; 7: 33–42. - "V体育安卓版" PubMed
-
- Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 2013; 19: 704–712. - "V体育官网入口" PMC - PubMed
-
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 2012; 8: 390–398. - PubMed
-
- Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 2013; 9: 721–730. - PubMed
MeSH terms
- V体育2025版 - Actions
- "V体育2025版" Actions
- "V体育2025版" Actions
- VSports - Actions
- VSports app下载 - Actions
Substances
- V体育安卓版 - Actions
- Actions (VSports app下载)
LinkOut - more resources
Full Text Sources
V体育2025版 - Other Literature Sources
Medical
"VSports手机版" Miscellaneous

