Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2 (VSports)
- PMID: 28270598
- PMCID: PMC5373368
- DOI: 10.1073/pnas.1621052114
Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2
"V体育ios版" Abstract
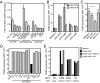
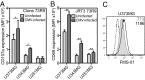
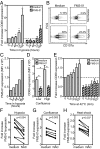
Human γδ T cells comprise a first line of defense through T-cell receptor (TCR) recognition of stressed cells. However, the molecular determinants and stress pathways involved in this recognition are largely unknown. Here we show that exposure of tumor cells to various stress situations led to tumor cell recognition by a Vγ8Vδ3 TCR. Using a strategy that we previously developed to identify antigenic ligands of γδ TCRs, annexin A2 was identified as the direct ligand of Vγ8Vδ3 TCR, and was found to be expressed on tumor cells upon the stress situations tested in a reactive oxygen species-dependent manner. Moreover, purified annexin A2 was able to stimulate the proliferation of a Vδ2neg γδ T-cell subset within peripheral blood mononuclear cells and other annexin A2-specific Vδ2neg γδ T-cell clones could be derived from peripheral blood mononuclear cells. We thus propose membrane exposure of annexin A2 as an oxidative stress signal for some Vδ2neg γδ T cells that could be involved in an adaptive stress surveillance VSports手机版. .
Keywords: cell stress surveillance; cytomegalovirus; gamma-delta T cells; innate-like lymphocytes; tumor immunology. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

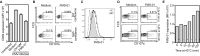
References (V体育平台登录)
-
- Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18(1):14–17. - PubMed (V体育官网)
-
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179(1):323–328. - "V体育ios版" PMC - PubMed
-
- Chien YH, Meyer C, Bonneville M. γδ T cells: First line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. - PubMed
-
- Born WK, Kemal Aydintug M, O’Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol. 2013;10(1):13–20. - "VSports注册入口" PMC - PubMed
-
- Crowley MP, et al. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287(5451):314–316. - PubMed
Publication types
- VSports手机版 - Actions
"V体育平台登录" MeSH terms
- "VSports注册入口" Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- "VSports最新版本" Actions
- V体育平台登录 - Actions
"VSports在线直播" Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

