"V体育官网入口" Mcl-1 Degradation Is Required for Targeted Therapeutics to Eradicate Colon Cancer Cells
- PMID: 28202514
- PMCID: "VSports app下载" PMC5626525
- DOI: 10.1158/0008-5472.CAN-16-3242
Mcl-1 Degradation Is Required for Targeted Therapeutics to Eradicate Colon Cancer Cells
Abstract (VSports)
The Bcl-2 family protein Mcl-1 is often degraded in cancer cells subjected to effective therapeutic treatment, and defective Mcl-1 degradation has been associated with intrinsic and acquired drug resistance. However, a causal relationship between Mcl-1 degradation and anticancer drug responses has not been directly established, especially in solid tumor cells where Mcl-1 inhibition alone is insufficient to trigger cell death VSports手机版. In this study, we present evidence that Mcl-1 participates directly in determining effective therapeutic responses in colon cancer cells. In this setting, Mcl-1 degradation was induced by a variety of multikinase inhibitor drugs, where it relied upon GSK3β phosphorylation and FBW7-dependent ubiquitination. Specific blockade by genetic knock-in (KI) abolished apoptotic responses and conferred resistance to kinase inhibitors. Mcl-1-KI also suppressed the antiangiogenic and anti-hypoxic effects of kinase inhibitors in the tumor microenvironment. Interestingly, these same inhibitors also induced the BH3-only Bcl-2 family protein PUMA, which is required for apoptosis. Degradation-resistant Mcl-1 bound and sequestered PUMA from other prosurvival proteins to maintain cell survival, which was abolished by small-molecule Mcl-1 inhibitors. Our findings establish a pivotal role for Mcl-1 degradation in the response of colon cancer cells to targeted therapeutics, and they provide a useful rational platform to develop Mcl-1-targeting agents that can overcome drug resistance. Cancer Res; 77(9); 2512-21. ©2017 AACR. .
©2017 American Association for Cancer Research. V体育安卓版.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures





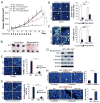
References
-
- Bhola PD, Letai A. Mitochondria-Judges and Executioners of Cell Death Sentences. Mol Cell. 2016;61:695–704. - PMC (VSports app下载) - PubMed
-
- Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–9. - PubMed
V体育平台登录 - Publication types
MeSH terms
- VSports最新版本 - Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- V体育2025版 - Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
- V体育安卓版 - Actions
VSports注册入口 - Substances
- Actions (V体育官网)
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- "VSports app下载" Actions
- "VSports注册入口" Actions
Grants and funding (V体育安卓版)
LinkOut - more resources
Full Text Sources (VSports在线直播)
Other Literature Sources

