Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems
- PMID: 28193861
- PMCID: PMC5338555 (VSports在线直播)
- DOI: 10.1073/pnas.1615771114
"V体育2025版" Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems
Abstract
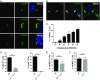
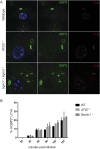
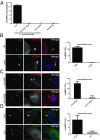
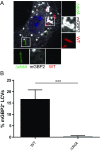
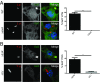
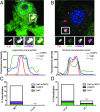
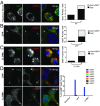
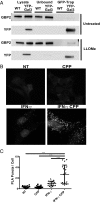
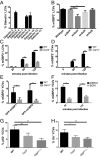
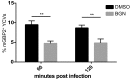
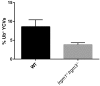
Many invasive bacteria establish pathogen-containing vacuoles (PVs) as intracellular niches for microbial growth. Immunity to these infections is dependent on the ability of host cells to recognize PVs as targets for host defense. The delivery of several host defense proteins to PVs is controlled by IFN-inducible guanylate binding proteins (GBPs), which themselves dock to PVs through poorly characterized mechanisms. Here, we demonstrate that GBPs detect the presence of bacterial protein secretion systems as "patterns of pathogenesis" associated with PVs. We report that the delivery of GBP2 to Legionella-containing vacuoles is dependent on the bacterial Dot/Icm secretion system, whereas the delivery of GBP2 to Yersinia-containing vacuoles (YCVs) requires hypersecretion of Yersinia translocon proteins. We show that the presence of bacterial secretion systems directs cytosolic carbohydrate-binding protein Galectin-3 to PVs and that the delivery of GBP1 and GBP2 to Legionella-containing vacuoles or YCVs is substantially diminished in Galectin-3-deficient cells. Our results illustrate that insertion of bacterial secretion systems into PV membranes stimulates Galectin-3-dependent recruitment of antimicrobial GBPs to PVs as part of a coordinated host defense program. VSports手机版.
Keywords: galectin; guanylate binding proteins; immunity-related GTPase; interferon; ubiquitin V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
VSports最新版本 - Figures

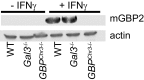
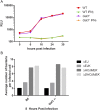
References
-
- Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev. 2015;265(1):85–102. - PubMed
Publication types
- VSports手机版 - Actions
"VSports" MeSH terms
- "VSports在线直播" Actions
- "V体育ios版" Actions
- VSports注册入口 - Actions
- V体育2025版 - Actions
- "V体育平台登录" Actions
- Actions (VSports)
Substances
- V体育官网入口 - Actions
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- V体育ios版 - Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

