"VSports" Capability of Neutrophils to Form NETs Is Not Directly Influenced by a CMA-Targeting Peptide
- PMID: 28191006
- PMCID: PMC5269539
- DOI: 10.3389/fimmu.2017.00016
Capability of Neutrophils to Form NETs Is Not Directly Influenced by a CMA-Targeting Peptide
Abstract
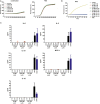
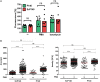
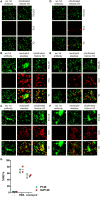
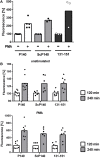
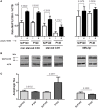
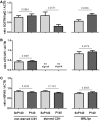
During inflammatory reaction, neutrophils exhibit numerous cellular and immunological functions, notably the formation of neutrophil extracellular traps (NETs) and autophagy. NETs are composed of decondensed chromatin fibers coated with various antimicrobial molecules derived from neutrophil granules. NETs participate in antimicrobial defense and can also display detrimental roles and notably trigger some of the immune features of systemic lupus erythematosus (SLE) and other autoimmune diseases. Autophagy is a complex and finely regulated mechanism involved in the cell survival/death balance that may be connected to NET formation. To shed some light on the connection between autophagy and NET formation, we designed a number of experiments in human neutrophils and both in normal and lupus-prone MRL/lpr mice to determine whether the synthetic peptide P140, which is capable of selectively modulating chaperone-mediated autophagy (CMA) in lymphocytes, could alter NET formation. P140/Lupuzor™ is currently being evaluated in phase III clinical trials involving SLE patients. Overall our in vitro and in vivo studies established that P140 does not influence NET formation, cytokine/chemokine production, or CMA in neutrophils VSports手机版. Thus, the beneficial effect of P140/Lupuzor™ in SLE is apparently not directly related to modulation of neutrophil function. .
Keywords: NET formation; P140/Lupuzor; autophagy; murine models of lupus; neutrophils; systemic lupus erythematosus V体育安卓版. .
Figures

References
-
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum (2002) 46(1):191–201.10.1002/1529-0131(200201)46:1<191:AID-ART10027>3.0.CO;2-K - DOI - PubMed
-
- Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A (2010) 107(21):9813–8.10.1073/pnas.0909927107 - DOI (VSports在线直播) - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

