Epithelial expression and function of trypsin-3 in irritable bowel syndrome
- PMID: 28096305
- PMCID: VSports手机版 - PMC5595105
- DOI: 10.1136/gutjnl-2016-312094 (V体育官网入口)
"VSports app下载" Epithelial expression and function of trypsin-3 in irritable bowel syndrome
VSports手机版 - Abstract
Objectives: Proteases are key mediators of pain and altered enteric neuronal signalling, although the types and sources of these important intestinal mediators are unknown VSports手机版. We hypothesised that intestinal epithelium is a major source of trypsin-like activity in patients with IBS and this activity signals to primary afferent and enteric nerves and induces visceral hypersensitivity. .
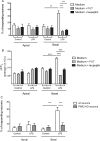
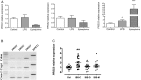
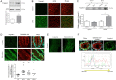
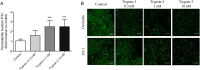
Design: Trypsin-like activity was determined in tissues from patients with IBS and in supernatants of Caco-2 cells stimulated or not V体育安卓版. These supernatants were also applied to cultures of primary afferents. mRNA isoforms of trypsin (PRSS1, 2 and 3) were detected by reverse transcription-PCR, and trypsin-3 protein expression was studied by western blot analysis and immunohistochemistry. Electrophysiological recordings and Ca2+ imaging in response to trypsin-3 were performed in mouse primary afferent and in human submucosal neurons, respectively. Visceromotor response to colorectal distension was recorded in mice administered intracolonically with trypsin-3. .
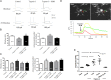
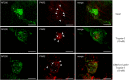
Results: We showed that stimulated intestinal epithelial cells released trypsin-like activity specifically from the basolateral side. This activity was able to activate sensory neurons V体育ios版. In colons of patients with IBS, increased trypsin-like activity was associated with the epithelium. We identified that trypsin-3 was the only form of trypsin upregulated in stimulated intestinal epithelial cells and in tissues from patients with IBS. Trypsin-3 was able to signal to human submucosal enteric neurons and mouse sensory neurons, and to induce visceral hypersensitivity in vivo, all by a protease-activated receptor-2-dependent mechanism. .
Conclusions: In IBS, the intestinal epithelium produces and releases the active protease trypsin-3, which is able to signal to enteric neurons and to induce visceral hypersensitivity VSports最新版本. .
Keywords: ABDOMINAL PAIN; IRRITABLE BOWEL SYNDROME; NERVE - GUT INTERACTIONS; TRYPSIN. V体育平台登录.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www. bmj. com/company/products-services/rights-and-licensing/ VSports注册入口. .
Conflict of interest statement
Competing interests: None declared.
Figures


VSports最新版本 - Comment in
-
VSports在线直播 - IBS: The power of protease activity in IBS.Nat Rev Gastroenterol Hepatol. 2017 Mar;14(3):139. doi: 10.1038/nrgastro.2017.14. Epub 2017 Feb 1. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28144027 No abstract available.
-
Identification of an epithelial member of the protease family, supporting the potential diagnostic and therapeutic role of serine proteases in IBS.Gut. 2017 Oct;66(10):1731-1732. doi: 10.1136/gutjnl-2016-313664. Epub 2017 Mar 10. Gut. 2017. PMID: 28283539 No abstract available.
References
-
- Sperber AD, Dumitrascu D, Fukudo S, et al. . The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut 2016. doi: 10.1136/gutjnl-2015-311240. [Epub ahead of print 27 Jan 2016] 10.1136/gutjnl-2015-311240 - DOI - PubMed
-
- Hyun E, Andrade-Gordon P, Steinhoff M, et al. . Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut 2008;57:1222–9. 10.1136/gut.2008.150722 - DOI (VSports手机版) - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- Actions (VSports在线直播)
- Actions (V体育安卓版)
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- V体育2025版 - Actions
- Actions (VSports注册入口)
- "VSports app下载" Actions
- "VSports" Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- "V体育平台登录" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
Substances (V体育安卓版)
- Actions (V体育官网)
- V体育官网 - Actions
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- V体育ios版 - Actions
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育官网)
"V体育2025版" Miscellaneous
