Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury
- PMID: 28087985
- PMCID: PMC5511097
- DOI: 10.1111/jgh.13731
V体育官网入口 - Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury
Abstract
Background and aim: Impaired gut-liver axis is a potential factor contributing to alcoholic liver disease VSports手机版. Ethanol depletes intestinal integrity and causes gut dysbiosis. Butyrate, a fermentation byproduct of gut microbiota, is altered negatively following chronic ethanol exposure. This study aimed to determine whether prophylactic tributyrin could protect the intestinal barrier and liver in mice during combined chronic-binge ethanol exposure. .
Methods: C57BL/6J mice exposed to 5% v/v ethanol-containing diet for 10 days received a single ethanol gavage (5 g/kg) 9 h before euthanasia. Control mice were isocalorically pair-fed maltose dextrin for ethanol. Diets were supplemented (5 mM) with tributyrin or glycerol. Intestine and liver disease activity was assessed histologically. Protein and mRNA expression of tight junction (TJ) proteins, toll-like receptors, and tumor necrosis factor-alpha were assessed. Caco-2 monolayers with or without ethanol exposure and/or sodium butyrate were used to test butyrate's direct effects on intestinal integrity V体育安卓版. .
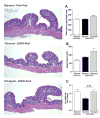
Results: Chronic-binge ethanol feeding impaired intestinal TJ protein co-localization staining; however, tributyrin co-treatment mitigated these effects. Ethanol depleted TJ and transepithelial electrical resistance in Caco-2 monolayers, but butyrate co-treatment reduced these effects. Hepatic toll-like receptor mRNA expression and tumor necrosis factor-alpha protein expression was induced by ethanol; however, the response was significantly dampened in mice co-treated with tributyrin. Tributyrin altered localization of both neutrophils and single hepatocyte death: Leukocytes and apoptotic hepatocytes localized predominantly around the portal tract in ethanol-only treated mice, whereas localization predominated around the central vein in ethanol-tributyrin mice. V体育ios版.
Conclusions: Prophylactic tributyrin supplementation mitigated effects of combined chronic-binge ethanol exposure on disruption of intestinal TJ localization and intestinal permeability and liver injury VSports最新版本. .
Keywords: alcoholic liver disease; chronic-binge ethanol; gut permeability; tight junction proteins; tributyrin V体育平台登录. .
© 2017 Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd VSports注册入口. .
V体育官网入口 - Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures (VSports app下载)





References
-
- Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. - "VSports最新版本" PMC - PubMed
-
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Quin X, Liu Y, et al. Metagenomic analysis of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLOS One. 2013;8:e53028. - PMC (VSports最新版本) - PubMed
MeSH terms
- "V体育2025版" Actions
- VSports最新版本 - Actions
- Actions (V体育官网)
- "VSports最新版本" Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
V体育ios版 - Substances
- "V体育官网" Actions
- VSports app下载 - Actions
- V体育安卓版 - Actions
- V体育ios版 - Actions
"VSports注册入口" Grants and funding
LinkOut - more resources
VSports在线直播 - Full Text Sources
Other Literature Sources

