Connective Tissue Growth Factor Promotes Pulmonary Epithelial Cell Senescence and Is Associated with COPD Severity
- PMID: 28026993
- PMCID: "V体育平台登录" PMC5362315
- DOI: 10.1080/15412555.2016.1262340
V体育平台登录 - Connective Tissue Growth Factor Promotes Pulmonary Epithelial Cell Senescence and Is Associated with COPD Severity
Abstract
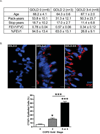
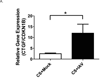
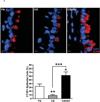
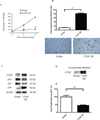
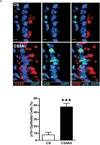
The purpose of this study was to determine whether expression of connective tissue growth factor (CTGF) protein in chronic obstructive pulmonary disease (COPD) is consistent in humans and animal models of COPD and to investigate the role of this protein in lung epithelial cells. CTGF in lung epithelial cells of ex-smokers with COPD was compared with ex-smokers without COPD by immunofluorescence. A total of twenty C57Bl/6 mice and sixteen non-human primates (NHPs) were exposed to cigarette smoke (CS) for 4 weeks. Ten mice of these CS-exposed mice and eight of the CS-exposed NHPs were infected with H3N2 influenza A virus (IAV), while the remaining ten mice and eight NHPs were mock-infected with vehicle as control. Both mRNA and protein expression of CTGF in lung epithelial cells of mice and NHPs were determined. The effects of CTGF overexpression on cell proliferation, p16 protein, and senescence-associated β-galactosidase (SA-β-gal) activity were examined in cultured human bronchial epithelial cells (HBECs). In humans, CTGF expression increased with increasing COPD severity. We found that protein expression of CTGF was upregulated in lung epithelial cells in both mice and NHPs exposed to CS and infected with IAV compared to those exposed to CS only. When overexpressed in HBECs, CTGF accelerated cellular senescence accompanied by p16 accumulation. Both CTGF and p16 protein expression in lung epithelia are positively associated with the severity of COPD in ex-smokers. These findings show that CTGF is consistently expressed in epithelial cells of COPD lungs VSports手机版. By accelerating lung epithelial senescence, CTGF may block regeneration relative to epithelial cell loss and lead to emphysema. .
Keywords: Airway epithelial cells; alveolar epithelial cells; cellular senescence; cigarette smoke; connective tissue growth factor; non-human primates. V体育安卓版.
Conflict of interest statement
All authors state there is no conflicts of interest.
Figures







References
-
- Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2004;101(41):14895–14900. - PMC (VSports app下载) - PubMed
-
- Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med. 1982;307(17):1042–1046. - PubMed
-
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. - PubMed
MeSH terms
- Actions (V体育官网)
- "V体育ios版" Actions
- VSports - Actions
- "VSports在线直播" Actions
- VSports手机版 - Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- V体育2025版 - Actions
- "VSports最新版本" Actions
- Actions (V体育2025版)
- "VSports" Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- VSports在线直播 - Actions
Substances
- VSports最新版本 - Actions
- VSports注册入口 - Actions
- VSports app下载 - Actions
- Actions (VSports)
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources (VSports)
Medical (VSports)
Miscellaneous
