Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion
- PMID: 27991918
- PMCID: V体育官网入口 - PMC5540654
- DOI: 10.1038/nm.4245
Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion (VSports app下载)
"VSports最新版本" Abstract
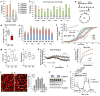
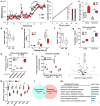
Type 2 diabetes and insulin resistance are associated with reduced glucose utilization in the muscle and poor exercise performance. Here we find that depletion of the epigenome modifier histone deacetylase 3 (HDAC3) specifically in skeletal muscle causes severe systemic insulin resistance in mice but markedly enhances endurance and resistance to muscle fatigue, despite reducing muscle force VSports手机版. This seemingly paradoxical phenotype is due to lower glucose utilization and greater lipid oxidation in HDAC3-depleted muscles, a fuel switch caused by the activation of anaplerotic reactions driven by AMP deaminase 3 (Ampd3) and catabolism of branched-chain amino acids. These findings highlight the pivotal role of amino acid catabolism in muscle fatigue and type 2 diabetes pathogenesis. Further, as genome occupancy of HDAC3 in skeletal muscle is controlled by the circadian clock, these results delineate an epigenomic regulatory mechanism through which the circadian clock governs skeletal muscle bioenergetics. These findings suggest that physical exercise at certain times of the day or pharmacological targeting of HDAC3 could potentially be harnessed to alter systemic fuel metabolism and exercise performance. .
Conflict of interest statement
The authors disclose no competing financial conflict of interest.
VSports手机版 - Figures




Comment in
-
HDAC3 sets the timer on muscle fuel switching.Nat Med. 2017 Feb 7;23(2):148-150. doi: 10.1038/nm.4282. Nat Med. 2017. PMID: 28170378 No abstract available.
References
-
- Brüning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 1998;2:559–569. - PubMed
MeSH terms
- Actions (V体育2025版)
- Actions (VSports)
- Actions (VSports app下载)
- Actions (VSports)
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- Actions (VSports)
- "VSports app下载" Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
- "VSports在线直播" Actions
V体育ios版 - Substances
- "V体育安卓版" Actions
- V体育2025版 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
Grants and funding
V体育平台登录 - LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育平台登录)
Molecular Biology Databases (V体育安卓版)

