Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model
- PMID: 27911853
- PMCID: PMC5356807
- DOI: 10.18632/oncotarget.13676
Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model (V体育安卓版)
Abstract
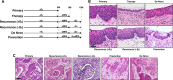
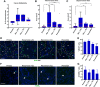
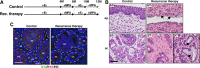
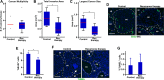
Studies using K14E6/K14E7 transgenic mice expressing E6 and E7 oncoprotein of human papillomavirus type 16 (HPV16) have demonstrated that estrogen (E2) is required for the genesis and growth of cervical cancer. Our prior study using the same mouse model has showed that progestin drug medroxyprogesterone acetate (MPA) promotes regression of primary cervical cancer. In the present study, we use the same transgenic mouse model to determine whether the cancer recurs after MPA therapy. Cervical cancer recurred even if MPA treatment was continued. Unlike primary cervical cancer, the cancer recurred even in the absence of exogenous E2 when MPA treatment was ceased VSports手机版. Furthermore, recurrent cervical cancer did not fully regress upon MPA treatment. Our results support that MPA fails to completely eliminate primary cervical cancer cells and that remaining cancer cells grow independent of exogenous E2 and are refractory to MPA. .
Keywords: cervical cancer; human papillomavirus (HPV); medroxyprogesterone acetate (MPA); recurrence; therapy resistance. V体育安卓版.
Conflict of interest statement
Authors have nothing to disclose.
Figures

"VSports手机版" References
-
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. - V体育ios版 - PubMed
-
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–60. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Downs LS, Smith JS, Scarinci I, Flowers L, Parham G. The disparity of cervical cancer in diverse populations. Gynecol Oncol. 2008;109:S22–30. - PubMed
MeSH terms
- Actions (V体育安卓版)
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- "V体育官网" Actions
- "V体育平台登录" Actions
- Actions (VSports)
- "VSports最新版本" Actions
- "VSports在线直播" Actions
- Actions (VSports)
Substances
- V体育2025版 - Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (V体育安卓版)

