Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps
- PMID: 27867387
- PMCID: PMC5095130
- DOI: "V体育2025版" 10.3389/fimmu.2016.00484
Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps
Abstract
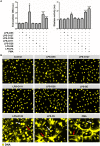
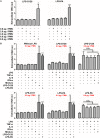
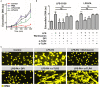
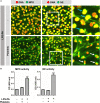
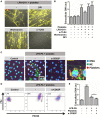
The release of neutrophil extracellular traps (NETs), either during "suicidal" or "vital" NETosis, represents an important strategy of neutrophils to combat Gram-negative bacteria. Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, is a reported stimulus for NET formation. Although it is widely acknowledged that the structural diversity in LPS structures can elicit heterogeneous immune responses, species- and serotype-specific differences in the capacity of LPS to trigger NET formation have not yet been investigated. In the present study, we compared the NET-inducing potential of LPS derived from Escherichia coli (serotypes O55:B5, O127:B8, O128:B12, O111:B4, and O26:B6), Salmonella enterica (serotype enteritidis), and Pseudomonas aeruginosa (serotype 10), under platelet-free and platelet-rich conditions in vitro, and in whole blood ex vivo. Here, we demonstrate that under serum- and platelet-free conditions, mimicking tissue circumstances, neutrophils discriminate between LPS of different bacterial sources and selectively release NETs only in response to LPS derived from E. coli O128:B12 and P. aeruginosa 10, which both induced "suicidal" NETosis in an autophagy- and reactive oxygen species (ROS)-dependent, but TLR4-independent manner. Intriguingly, in whole blood cultures ex vivo, or in vitro in the presence of platelets, all LPS serotypes induced "vital" NET formation. This platelet-dependent release of NETs occurred rapidly without neutrophil cell death and was independent from ROS formation and autophagy but required platelet TLR4 and CD62P-dependent platelet-neutrophil interactions VSports手机版. Taken together, our data reveal a complex interplay between neutrophils and LPS, which can induce both "suicidal" and "vital" NETosis, depending on the bacterial origin of LPS and the presence or absence of platelets. Our findings suggest that LPS sensing by neutrophils may be a critical determinant for restricting NET release to certain Gram-negative bacteria only, which in turn may be crucial for minimizing unnecessary NET-associated immunopathology. .
Keywords: NETosis; cell death; lipopolysaccharides; neutrophil extracellular traps; platelets V体育安卓版. .
Figures


References
-
- Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol (2012) 198:773–83.10.1083/jcb.201203170 - "VSports最新版本" DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports app下载)

